Mechanical and in vitro evaluation of an experimental canine patent ductus arteriosus occlusion device
- PMID: 26766327
- PMCID: PMC5821254
- DOI: 10.1016/j.jmbbm.2015.12.014
Mechanical and in vitro evaluation of an experimental canine patent ductus arteriosus occlusion device
Abstract
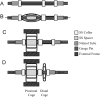
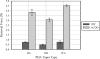
Patent ductus arteriosus (PDA) is a congenital cardiovascular malformation in which a fetal connection between the aorta and pulmonary artery remains patent after birth. This defect commonly results in clinical complications, even death, necessitating closure. Surgical ligation is the most common treatment but requires a thoracotomy and is therefore invasive. A minimally invasive option is preferable. A prototype device for PDA occlusion which utilizes shape memory polymer foams has been developed and evaluated using mechanical and in vitro experiments. Removal force and radial pressure measurements show that the prototype device exhibited a lower removal force and radial pressure than a commercially available device. The in vitro experiments conducted within simplified and physiological PDA models showed that the prototype does not migrate out of position into the pulmonary artery at either physiological or elevated pressures in multiple model configurations. While the radial pressure and removal force were lower than commercial devices, the device performed acceptably in the in vitro benchtop experiments warranting further prototype development.
Keywords: ACDO; Patent ductus arteriosus; Shape memory polymer.
Copyright © 2016. Published by Elsevier Ltd.
Figures









Similar articles
-
An experimental canine patent ductus arteriosus occlusion device based on shape memory polymer foam in a nitinol cage.J Mech Behav Biomed Mater. 2017 Nov;75:279-292. doi: 10.1016/j.jmbbm.2017.07.033. Epub 2017 Jul 25. J Mech Behav Biomed Mater. 2017. PMID: 28759840 Free PMC article.
-
In vitro evaluation of mechanical characteristics of a novel Patent Ductus Arteriosus (PDA) occlusion device with a new lateral pressure measurement study.J Mech Behav Biomed Mater. 2010 Oct;3(7):520-9. doi: 10.1016/j.jmbbm.2010.05.009. Epub 2010 Jun 8. J Mech Behav Biomed Mater. 2010. PMID: 20696417
-
Patent ductus arteriosus occlusion in small dogs utilizing a low profile Amplatz® canine duct occluder prototype.J Vet Cardiol. 2015 Sep;17(3):203-9. doi: 10.1016/j.jvc.2015.06.002. Epub 2015 Sep 9. J Vet Cardiol. 2015. PMID: 26363940
-
The patent ductus arteriosus in term infants, children, and adults.Semin Perinatol. 2012 Apr;36(2):146-53. doi: 10.1053/j.semperi.2011.09.025. Semin Perinatol. 2012. PMID: 22414886 Review.
-
Transarterial coil embolization for canine patent ductus arteriosus occlusion.Clin Tech Small Anim Pract. 2005 Aug;20(3):196-202. doi: 10.1053/j.ctsap.2005.05.008. Clin Tech Small Anim Pract. 2005. PMID: 16180402 Review.
Cited by
-
Star-PCL shape memory polymer (SMP) scaffolds with tunable transition temperatures for enhanced utility.J Mater Chem B. 2024 Apr 17;12(15):3694-3702. doi: 10.1039/d4tb00050a. J Mater Chem B. 2024. PMID: 38529581 Free PMC article.
-
Shape memory polymer (SMP) scaffolds with improved self-fitting properties.J Mater Chem B. 2021 May 14;9(18):3826-3837. doi: 10.1039/d0tb02987d. Epub 2021 Apr 15. J Mater Chem B. 2021. PMID: 33979417 Free PMC article.
-
Micro-CT and histopathology methods to assess host response of aneurysms treated with shape memory polymer foam-coated coils versus bare metal coil occlusion devices.J Biomed Mater Res B Appl Biomater. 2020 Jul;108(5):2238-2249. doi: 10.1002/jbm.b.34561. Epub 2020 Jan 21. J Biomed Mater Res B Appl Biomater. 2020. PMID: 31961062 Free PMC article.
-
An experimental canine patent ductus arteriosus occlusion device based on shape memory polymer foam in a nitinol cage.J Mech Behav Biomed Mater. 2017 Nov;75:279-292. doi: 10.1016/j.jmbbm.2017.07.033. Epub 2017 Jul 25. J Mech Behav Biomed Mater. 2017. PMID: 28759840 Free PMC article.
References
-
- Achen SE, Miller MW, Gordon SG, Saunders AB, Roland RM, Drourr LT. Transarterial ductal occlusion with the amplatzer vascular plug in 31 Dogs. J Vet Intern Med. 2008;22:1348–1352. - PubMed
-
- Birchard SJ, Bonagura JD, Fingland RB. Results of ligation of patent ductus-arteriosus in Dogs – 201 Cases (1969–1988) J Am Vet Med Assoc. 1990;196:2011–2013. - PubMed
-
- Bureau SP, Monnet E, Orton EC. Evaluation of survival rate and prognostic indicators for surgical treatment of left-to-right patent ductus arteriosus in dogs: 52 cases (1995–2003) J Am Vet Med Assoc. 2005;227:1794–1799. - PubMed
-
- Campbell FE, Thomas WP, Miller SJ, Berger D, Kittleson MD. Immediate and late outcomes of transarterial coil occlusion of patent ductus arteriosus in dogs. J Vet Intern Med. 2006;20:83–96. - PubMed
-
- Coceani F, Olley PM. The response of the ductus arteriosus to prostaglandins. Can J Physiol Pharm. 1973;51:220–225. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

