Broadly Neutralizing Antibody Responses in a Large Longitudinal Sub-Saharan HIV Primary Infection Cohort
- PMID: 26766578
- PMCID: PMC4713061
- DOI: 10.1371/journal.ppat.1005369
Broadly Neutralizing Antibody Responses in a Large Longitudinal Sub-Saharan HIV Primary Infection Cohort
Abstract
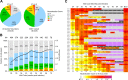
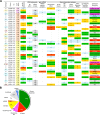
Broadly neutralizing antibodies (bnAbs) are thought to be a critical component of a protective HIV vaccine. However, designing vaccines immunogens able to elicit bnAbs has proven unsuccessful to date. Understanding the correlates and immunological mechanisms leading to the development of bnAb responses during natural HIV infection is thus critical to the design of a protective vaccine. The IAVI Protocol C program investigates a large longitudinal cohort of primary HIV-1 infection in Eastern and South Africa. Development of neutralization was evaluated in 439 donors using a 6 cross-clade pseudo-virus panel predictive of neutralization breadth on larger panels. About 15% of individuals developed bnAb responses, essentially between year 2 and year 4 of infection. Statistical analyses revealed no influence of gender, age or geographical origin on the development of neutralization breadth. However, cross-clade neutralization strongly correlated with high viral load as well as with low CD4 T cell counts, subtype-C infection and HLA-A*03(-) genotype. A correlation with high overall plasma IgG levels and anti-Env IgG binding titers was also found. The latter appeared not associated with higher affinity, suggesting a greater diversity of the anti-Env responses in broad neutralizers. Broadly neutralizing activity targeting glycan-dependent epitopes, largely the N332-glycan epitope region, was detected in nearly half of the broad neutralizers while CD4bs and gp41-MPER bnAb responses were only detected in very few individuals. Together the findings suggest that both viral and host factors are critical for the development of bnAbs and that the HIV Env N332-glycan supersite may be a favorable target for vaccine design.
Conflict of interest statement
PPh and TW are employed by a commercial company, Monogram Biosciences, Inc. This affiliation does not alter our adherence to all the PLOS Pathogens policies on sharing data and materials. The other authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

