New Amniotic Membrane Based Biocomposite for Future Application in Reconstructive Urology
- PMID: 26766636
- PMCID: PMC4713072
- DOI: 10.1371/journal.pone.0146012
New Amniotic Membrane Based Biocomposite for Future Application in Reconstructive Urology
Abstract
Objective: Due to the capacity of the amniotic membrane (Am) to support re-epithelisation and inhibit scar formation, Am has a potential to become a considerable asset for reconstructive urology i.e., reconstruction of ureters and urethrae. The application of Am in reconstructive urology is limited due to a poor mechanical characteristic. Am reinforcement with electrospun nanofibers offers a new strategy to improve Am mechanical resistance, without affecting its unique bioactivity profile. This study evaluated biocomposite material composed of Am and nanofibers as a graft for urinary bladder augmentation in a rat model.
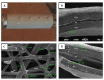
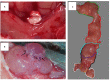
Material and methods: Sandwich-structured biocomposite material was constructed from frozen Am and covered on both sides with two-layered membranes prepared from electrospun poly-(L-lactide-co-E-caprolactone) (PLCL). Wistar rats underwent hemicystectomy and bladder augmentation with the biocomposite material.
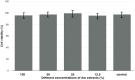
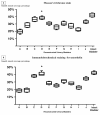
Results: Immunohistohemical analysis (hematoxylin and eosin [H&E], anti-smoothelin and Masson's trichrome staining [TRI]) revealed effective regeneration of the urothelial and smooth muscle layers. Anti-smoothelin staining confirmed the presence of contractile smooth muscle within a new bladder wall. Sandwich-structured biocomposite graft material was designed to regenerate the urinary bladder wall, fulfilling the requirements for normal bladder tension, contraction, elasticity and compliance. Mechanical evaluation of regenerated bladder wall conducted based on Young's elastic modulus reflected changes in the histological remodeling of the augmented part of the bladder. The structure of the biocomposite material made it possible to deliver an intact Am to the area for regeneration. An unmodified Am surface supported regeneration of the urinary bladder wall and the PLCL membranes did not disturb the regeneration process.
Conclusions: Am reinforcement with electrospun nanofibers offers a new strategy to improve Am mechanical resistance without affecting its unique bioactivity profile.
Conflict of interest statement
Figures




References
-
- Witjes JA, Compérat E, Cowan NC, De Santis M, Gakis G, Lebrét T, et al. Guidelines on muscle-invasive and metastatic bladder cancer. In: EAU Guidelines, edition presented at the 28th EAU Annual Congress, Milan 2013. ISBN 978-90-79754-71-7
-
- Levine LA, Strom KH, Lux MM. Buccal mucosa graft urethroplasty for anteriorurethral stricture repair: evaluation of the impact of stricture location and lichen sclerosus on surgical outcome. J Urol. 2007: 178 2011–5. - PubMed
-
- Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet 2006: 367 1241–6. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

