Genetic lineage tracing discloses arteriogenesis as the main mechanism for collateral growth in the mouse heart
- PMID: 26768261
- PMCID: PMC4752045
- DOI: 10.1093/cvr/cvw005
Genetic lineage tracing discloses arteriogenesis as the main mechanism for collateral growth in the mouse heart
Abstract
Aims: Capillary and arterial endothelial cells share many common molecular markers in both the neonatal and adult hearts. Herein, we aim to establish a genetic tool that distinguishes these two types of vessels in order to determine the cellular mechanism underlying collateral artery formation.
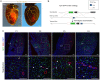
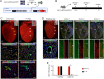
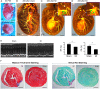
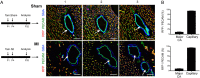
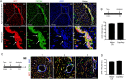
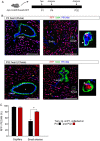
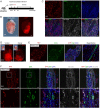
Methods and results: Using Apln-GFP and Apln-LacZ reporter mice, we demonstrate that APLN expression is enriched in coronary vascular endothelial cells. However, APLN expression is reduced in coronary arterial endothelial cells. Genetic lineage tracing, using an Apln-CreER mouse line, robustly labelled capillary endothelial cells, but not arterial endothelial cells. We leveraged this differential activity of Apln-CreER to study collateral artery formation following myocardial infarction (MI). In a neonatal heart MI model, we found that Apln-CreER-labelled capillary endothelial cells do not contribute to the large collateral arteries. Instead, these large collateral arteries mainly arise from pre-existing, infrequently labelled coronary arteries, indicative of arteriogenesis. Furthermore, in an adult heart MI model, Apln-CreER activity also distinguishes large and small diameter arteries from capillaries. Lineage tracing in this setting demonstrated that most large and small coronary arteries in the infarcted myocardium and border region are derived not from capillaries, but from pre-existing arteries.
Conclusion: Apln-CreER-mediated lineage tracing distinguishes capillaries from large arteries, in both the neonatal and adult hearts. Through genetic fate mapping, we demonstrate that pre-existing arteries, but not capillaries, extensively contribute to collateral artery formation following myocardial injury. These results suggest that arteriogenesis is the major mechanism underlying collateral vessel formation.
Keywords: Apln-CreER; Arterialization; Arteriogenesis; Collateral artery; Lineage tracing.
© The Author 2016. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures
References
-
- Helisch A, Schaper W. Arteriogenesis: the development and growth of collateral arteries. Microcirculation 2003;10:83–97. - PubMed
-
- Ito W, Arras M, Winkler B, Scholz D, Schaper J, Schaper W. Monocyte chemotactic protein-1 increases collateral and peripheral conductance after femoral artery occlusion. Circ Res 1997;80:829–837. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

