Improved survival of porcine acute liver failure by a bioartificial liver device implanted with induced human functional hepatocytes
- PMID: 26768767
- PMCID: PMC4746613
- DOI: 10.1038/cr.2016.6
Improved survival of porcine acute liver failure by a bioartificial liver device implanted with induced human functional hepatocytes
Erratum in
-
Author Correction: Improved survival of porcine acute liver failure by a bioartificial liver device implanted with induced human functional hepatocytes.Cell Res. 2025 Jun;35(6):469-470. doi: 10.1038/s41422-025-01108-5. Cell Res. 2025. PMID: 40148490 Free PMC article. No abstract available.
Abstract
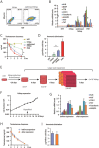
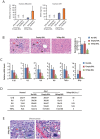
Acute liver failure (ALF) is a life-threatening illness. The extracorporeal cell-based bioartificial liver (BAL) system could bridge liver transplantation and facilitate liver regeneration for ALF patients by providing metabolic detoxification and synthetic functions. Previous BAL systems, based on hepatoma cells and non-human hepatocytes, achieved limited clinical advances, largely due to poor hepatic functions, cumbersome preparation or safety concerns of these cells. We previously generated human functional hepatocytes by lineage conversion (hiHeps). Here, by improving functional maturity of hiHeps and producing hiHeps at clinical scales (3 billion cells), we developed a hiHep-based BAL system (hiHep-BAL). In a porcine ALF model, hiHep-BAL treatment restored liver functions, corrected blood levels of ammonia and bilirubin, and prolonged survival. Importantly, human albumin and α-1-antitrypsin were detectable in hiHep-BAL-treated ALF pigs. Moreover, hiHep-BAL treatment led to attenuated liver damage, resolved inflammation and enhanced liver regeneration. Our findings indicate a promising clinical application of the hiHep-BAL system.
Figures

Similar articles
-
Reversal of liver failure using a bioartificial liver device implanted with clinical-grade human-induced hepatocytes.Cell Stem Cell. 2023 May 4;30(5):617-631.e8. doi: 10.1016/j.stem.2023.03.013. Epub 2023 Apr 13. Cell Stem Cell. 2023. PMID: 37059100
-
An extracorporeal bioartificial liver embedded with 3D-layered human liver progenitor-like cells relieves acute liver failure in pigs.Sci Transl Med. 2020 Jul 8;12(551):eaba5146. doi: 10.1126/scitranslmed.aba5146. Sci Transl Med. 2020. PMID: 32641490
-
Functional Evaluation of a Bioartificial Liver Support System Using Immobilized Hepatocyte Spheroids in a Porcine Model of Acute Liver Failure.Sci Rep. 2017 Jun 19;7(1):3804. doi: 10.1038/s41598-017-03424-2. Sci Rep. 2017. PMID: 28630420 Free PMC article.
-
Liver support therapy: an overview of the AMC-bioartificial liver research.Dig Surg. 2005;22(4):254-64. doi: 10.1159/000088055. Epub 2005 Sep 20. Dig Surg. 2005. PMID: 16174982 Review.
-
Bioartificial liver support systems for acute liver failure: A systematic review and meta-analysis of the clinical and preclinical literature.World J Gastroenterol. 2019 Jul 21;25(27):3634-3648. doi: 10.3748/wjg.v25.i27.3634. World J Gastroenterol. 2019. PMID: 31367162 Free PMC article.
Cited by
-
Liver regeneration after injury: Mechanisms, cellular interactions and therapeutic innovations.Clin Transl Med. 2024 Aug;14(8):e1812. doi: 10.1002/ctm2.1812. Clin Transl Med. 2024. PMID: 39152680 Free PMC article. Review.
-
Inventing Engineered Organoids for end-stage liver failure patients.J Mol Histol. 2022 Aug;53(4):611-621. doi: 10.1007/s10735-022-10085-7. Epub 2022 Jul 27. J Mol Histol. 2022. PMID: 35882727 Free PMC article. Review.
-
Mouse decellularised liver scaffold improves human embryonic and induced pluripotent stem cells differentiation into hepatocyte-like cells.PLoS One. 2017 Dec 20;12(12):e0189586. doi: 10.1371/journal.pone.0189586. eCollection 2017. PLoS One. 2017. PMID: 29261712 Free PMC article.
-
Cryopreserved cGMP-compliant human pluripotent stem cell-derived hepatic progenitors rescue mice from acute liver failure through rapid paracrine effects on liver cells.Stem Cell Res Ther. 2024 Mar 12;15(1):71. doi: 10.1186/s13287-024-03673-9. Stem Cell Res Ther. 2024. PMID: 38475825 Free PMC article.
-
A serum-free medium suitable for maintaining cell morphology and liver-specific function in induced human hepatocytes.Cytotechnology. 2019 Feb;71(1):329-344. doi: 10.1007/s10616-018-0289-2. Epub 2019 Jan 2. Cytotechnology. 2019. PMID: 30603919 Free PMC article.
References
-
- 1Bernal W, Wendon J. Acute liver failure. N Engl J Med 2013; 369:2525–2534. - PubMed
-
- 2Struecker B, Raschzok N, Sauer IM. Liver support strategies: cutting-edge technologies. Nat Rev Gastroenterol Hepatol 2014; 11:166–176. - PubMed
-
- 3Sussman NL, Kelly JH. Artificial liver. Clin Gastroenterol Hepatol 2014; 12:1439–1442. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

