Toward precision medicine for preserving fertility in cancer patients: existing and emerging fertility preservation options for women
- PMID: 26768785
- PMCID: PMC4717227
- DOI: 10.3802/jgo.2016.27.e22
Toward precision medicine for preserving fertility in cancer patients: existing and emerging fertility preservation options for women
Abstract
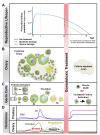
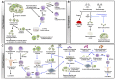
As the number of young cancer survivors increases, quality of life after cancer treatment is becoming an ever more important consideration. According to a report from the American Cancer Society, approximately 810,170 women were diagnosed with cancer in 2015 in the United States. Among female cancer survivors, 1 in 250 are of reproductive age. Anticancer therapies can result in infertility or sterility and can have long-term negative effects on bone health, cardiovascular health as a result of reproductive endocrine function. Fertility preservation has been identified by many young patients diagnosed with cancer as second only to survival in terms of importance. The development of fertility preservation technologies aims to help patients diagnosed with cancer to preserve or protect their fertility prior to exposure to chemo- or radiation therapy, thus improving their chances of having a family and enhancing their quality of life as a cancer survivor. Currently, sperm, egg, and embryo banking are standard of care for preserving fertility for reproductive-age cancer patients; ovarian tissue cryopreservation is still considered experimental. Adoption and surrogate may also need to be considered. All patients should receive information about the fertility risks associated with their cancer treatment and the fertility preservation options available in a timely manner, whether or not they decide to ultimately pursue fertility preservation. Because of the ever expanding number of options for treating cancer and preserving fertility, there is now an opportunity to take a precision medicine approach to informing patients about the fertility risks associated with their cancer treatment and the fertility preservation options that are available to them.
Keywords: Cancer; Chemo- or radiation therapy; Fertility Preservation; Oocyte; Precision Medicine; Technologies.
Conflict of interest statement
Figures
Comment in
-
Embryos need a cozy house.J Gynecol Oncol. 2016 May;27(3):e34. doi: 10.3802/jgo.2016.27.e34. J Gynecol Oncol. 2016. PMID: 27029754 Free PMC article. No abstract available.
-
Ovary is necessary to the health of uterus.J Gynecol Oncol. 2016 May;27(3):e35. doi: 10.3802/jgo.2016.27.e35. J Gynecol Oncol. 2016. PMID: 27029755 Free PMC article. No abstract available.
References
-
- Baker TG. A quantitative and cytological study of germ cells in human ovaries. Proc R Soc Lond B Biol Sci. 1963;158:417–433. - PubMed
-
- Mamsen LS, Lutterodt MC, Andersen EW, Byskov AG, Andersen CY. Germ cell numbers in human embryonic and fetal gonads during the first two trimesters of pregnancy: analysis of six published studies. Hum Reprod. 2011;26:2140–2145. - PubMed
-
- Faddy MJ, Gosden RG. A model conforming the decline in follicle numbers to the age of menopause in women. Hum Reprod. 1996;11:1484–1486. - PubMed
-
- Morgan S, Anderson RA, Gourley C, Wallace WH, Spears N. How do chemotherapeutic agents damage the ovary? Hum Reprod Update. 2012;18:525–535. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

