Replication characteristics of equine herpesvirus 1 and equine herpesvirus 3: comparative analysis using ex vivo tissue cultures
- PMID: 26768993
- PMCID: PMC4714513
- DOI: 10.1186/s13567-016-0305-5
Replication characteristics of equine herpesvirus 1 and equine herpesvirus 3: comparative analysis using ex vivo tissue cultures
Abstract
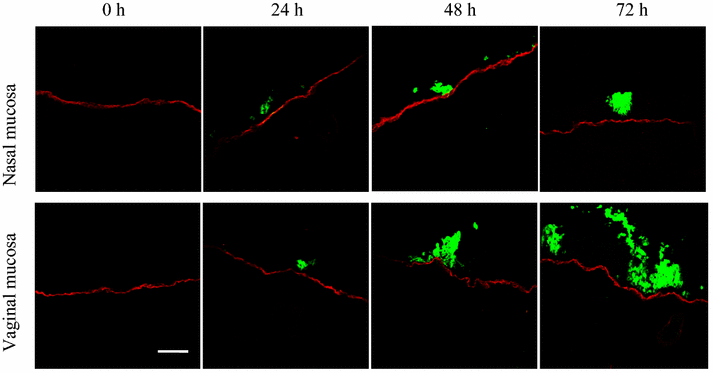
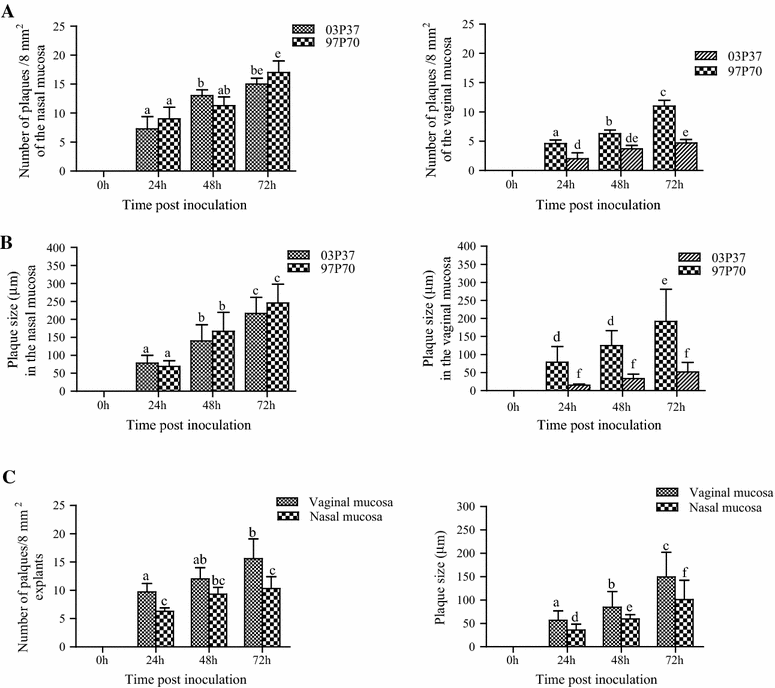
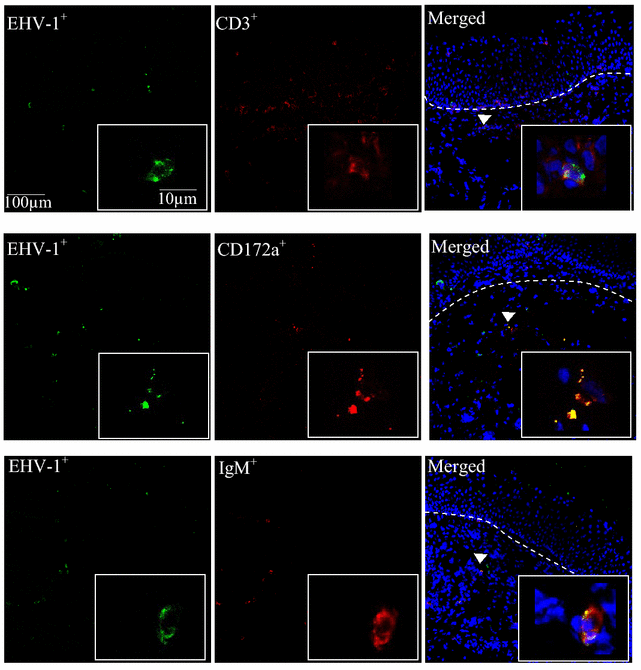
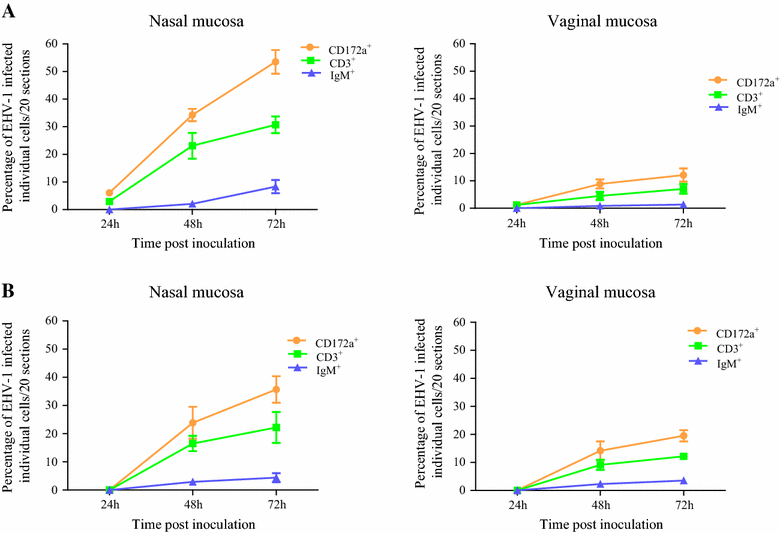
Replication kinetics and invasion characteristics of equine herpesvirus-1 and -3 (EHV-1/-3) in nasal and vaginal mucosae were compared using explants. The explants were cultured during 96 h with little change in viability. The tissues were inoculated with EHV-1 03P37 (neuropathogenic), 97P70 (abortigenic) and EHV-3 04P57, collected at 0, 24, 48 and 72 h post inoculation (pi) and stained for viral antigens. Both EHV-1 and EHV-3 replicated in a plaquewise manner. The plaques were already observed at 24 h pi, their size increased over time and did not directly cross the basement membrane (BM). However, EHV-1 infected the monocytic cells (MC) and hijacked these cells to invade the lamina propria. In contrast, EHV-3 replication was fully restricted to epithelial cells; the virus did not breach the BM via a direct cell-to-cell spread nor used infected MC. EHV-1-induced plaques were larger in nasal mucosa compared to vaginal mucosa. The opposite was found for EHV-3-induced plaques. Both EHV-1 strains replicated with comparable kinetics in nasal mucosa. However, the extent of replication of the abortigenic strain in vaginal mucosa was significantly higher than that of the neuropathogenic strain. Two-to-five-fold lower numbers of EHV-1-infected MC underneath the BM were found in vaginal mucosa than in nasal mucosa. Our study has shown that (i) EHV-1 has developed in evolution a predisposition for respiratory mucosa and EHV-3 for vaginal mucosa, (ii) abortigenic EHV-1 replicates better in vaginal mucosa than neuropathogenic EHV-1 and (iii) EHV-3 demonstrated a strict epithelial tropism whereas EHV-1 in addition hijacked MC to invade the lamina propria.
Figures

References
-
- Allen GP, Kydd JH, Slater JD, Smith KC. Equid herpesvirus 1 and equid herpesvirus 4 infections. In: Coetzer JAW, Tustin RC, editors. Infectious diseases of livestock. Cape Town: Oxford Press; 2004. pp. 829–859.
-
- Allen GP, Umphenour NW. Equine Coital Exanthema. In: Coetzer JAW, Tustin RC, editors. Infectious diseases of livestock. Cape Town: Oxford Press; 2004. pp. 860–867.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

