Full Expression of Cardiomyopathy Is Partly Dependent on B-Cells: A Pathway That Involves Cytokine Activation, Immunoglobulin Deposition, and Activation of Apoptosis
- PMID: 26769625
- PMCID: PMC4859365
- DOI: 10.1161/JAHA.115.002484
Full Expression of Cardiomyopathy Is Partly Dependent on B-Cells: A Pathway That Involves Cytokine Activation, Immunoglobulin Deposition, and Activation of Apoptosis
Abstract
Background: Limited information exists on the role of B-cell-dependent mechanisms in the progression of heart failure (HF). However, in failing human myocardium, there is evidence of deposition of activated complement components as well as anticardiac antibodies. We aimed to determine the contribution of B-cells in HF progression using a nonsurgical mouse model of nonischemic cardiomyopathy (CMP).
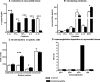
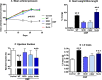
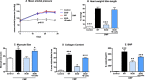
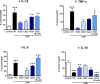
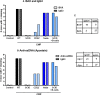
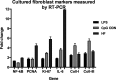
Methods and results: CMP protocol involved the use of l-NAME and NaCl in the drinking water and angiotensin-II infusion for 35 days. At day 35, mice were analyzed by cardiac magnetic resonance imaging, gene expression, and histology. Mice (12 weeks old) were divided into 4 groups, all in C57BL/6 background: wild-type (WT) CMP; severe combined immunodeficiency (SCID) CMP (T- and B-cell deficient); CD22(-) CMP (B-cell depleted); and Nude CMP (T-cell deficient), with their respective controls. We performed B-cell depletion and reconstitution protocols. The protective effect of B-cell depletion was demonstrated by a significant reduction of cell hypertrophy and collagen deposition and a preserved ejection fraction in the CD22(-) CMP group compared to WT CMP. Once SCID mice underwent B-cell reconstitution with isolated CMP B-cells, the CMP phenotype was restored. Furthermore, deposition of IgG3 and apoptosis in the myocardium follows the development of CMP; in addition, in vitro studies demonstrated that activated B-cells stimulate collagen production by cardiac fibroblasts.
Conclusions: The absence of B-cells in this model of HF resulted in less hypertrophy and collagen deposition, preservation of left ventricular function, and, in association with these changes, a reduction in expression of proinflammatory cytokines, immunoglobulin G deposition, and apoptosis in the myocardium. Taken together, these data suggest that B-cells play a contributory role in an angiotensin-II-induced HF model.
Keywords: antibodies; cardiomyopathy; immune system; lymphocytes; remodeling.
© 2016 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley Blackwell.
Figures


References
-
- Suzuki H, Sato R, Sato T, Shoji M, Iso Y, Kondo T, Shibata M, Koba S, Katagiri T. Time course of changes in the levels of interleukin 6 in acutely decompensated heart failure. Int J Cardiol. 2005;100:415–420. - PubMed
-
- Milani RV, Mehra MR, Endres S, Eigler A, Cooper ES, Lavie CJ Jr, Ventura HO. The clinical relevance of circulating tumor necrosis factor‐alpha in acute decompensated chronic heart failure without cachexia. Chest. 1996;110:992–995. - PubMed
-
- Peschel T, Schonauer M, Thiele H, Anker SD, Schuler G, Niebauer J. Invasive assessment of bacterial endotoxin and inflammatory cytokines in patients with acute heart failure. Eur J Heart Fail. 2003;5:609–614. - PubMed
-
- Torre‐Amione G, Orrego CM, Khalil N, Kottner‐Assad C, Leveque C, Celis R, Youker KA, Estep JD. Therapeutic plasma exchange a potential strategy for patients with advanced heart failure. J Clin Apher. 2010;25:323–330. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

