Mobile Phone Text Messages to Support Treatment Adherence in Adults With High Blood Pressure (SMS-Text Adherence Support [StAR]): A Single-Blind, Randomized Trial
- PMID: 26769742
- PMCID: PMC4750295
- DOI: 10.1161/CIRCULATIONAHA.115.017530
Mobile Phone Text Messages to Support Treatment Adherence in Adults With High Blood Pressure (SMS-Text Adherence Support [StAR]): A Single-Blind, Randomized Trial
Abstract
Background: We assessed the effect of automated treatment adherence support delivered via mobile phone short message system (SMS) text messages on blood pressure.
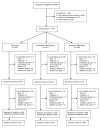
Methods and results: In this pragmatic, single-blind, 3-arm, randomized trial (SMS-Text Adherence Support [StAR]) undertaken in South Africa, patients treated for high blood pressure were randomly allocated in a 1:1:1 ratio to information only, interactive SMS text messaging, or usual care. The primary outcome was change in systolic blood pressure at 12 months from baseline measured with a validated oscillometric device. All trial staff were masked to treatment allocation. Analyses were intention to treat. Between June 26, 2012, and November 23, 2012, 1372 participants were randomized to receive information-only SMS text messages (n=457), interactive SMS text messages (n=458), or usual care (n=457). Primary outcome data were available for 1256 participants (92%). At 12 months, the mean adjusted change in systolic blood pressure compared with usual care was -2.2 mm Hg (95% confidence interval, -4.4 to -0.04) with information-only SMS and -1.6 mm Hg (95% confidence interval, -3.7 to 0.6) with interactive SMS. Odds ratios for the proportion of participants with a blood pressure <140/90 mm Hg were 1.42 (95% confidence interval, 1.03-1.95) for information-only messaging and 1.41 (95% confidence interval, 1.02-1.95) for interactive messaging compared with usual care.
Conclusions: In this randomized trial of an automated adherence support program delivered by SMS text message in a general outpatient population of adults with high blood pressure, we found a small reduction in systolic blood pressure control compared with usual care at 12 months. There was no evidence that an interactive intervention increased this effect.
Clinical trial registration: URL: http://www.clinicaltrials.gov. Unique identifier: NCT02019823. South African National Clinical Trials Register, number SANCTR DOH-27-1212-386; Pan Africa Trial Register, number PACTR201411000724141.
Keywords: behavior therapy; delivery of health care; patient compliance; randomized controlled trial; self care.
© 2016 American Heart Association, Inc.
Figures
Comment in
-
Text Messaging and Patient Engagement in an Increasingly Mobile World.Circulation. 2016 Feb 9;133(6):555-6. doi: 10.1161/CIRCULATIONAHA.116.021182. Epub 2016 Jan 14. Circulation. 2016. PMID: 26769741 No abstract available.
References
-
- Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–1360. - PubMed
-
- Lawes CMM, Vander Hoorn S, Rodgers A, for the International Society of Hypertension Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371:1513–1518. - PubMed
-
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, for the Prospective Studies Collaboration Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. - PubMed
-
- Yusuf S. Unresolved Issues in the Management of Hypertension. BMJ. 2010;55:832–834. - PubMed
-
- Chowdhury R, Khan H, Heydon E, Shroufi A, Fahimi S, Moore C, Stricker B, Mendis S, Hofman A, Mant J, Franco OH. Adherence to cardiovascular therapy: a meta-analysis of prevalence and clinical consequences. Eur Heart J. 2013;34:2940–8. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical