DANGER is involved in high glucose-induced radioresistance through inhibiting DAPK-mediated anoikis in non-small cell lung cancer
- PMID: 26769850
- PMCID: PMC4872778
- DOI: 10.18632/oncotarget.6887
DANGER is involved in high glucose-induced radioresistance through inhibiting DAPK-mediated anoikis in non-small cell lung cancer
Abstract
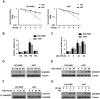
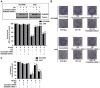
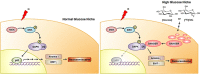
18F-labeled fluorodeoxyglucose (FDG) uptake during FDG positron emission tomography seems to reflect increased radioresistance. However, the exact molecular mechanism underlying high glucose (HG)-induced radioresistance is unclear. In the current study, we showed that ionizing radiation-induced activation of the MEK-ERK-DAPK-p53 signaling axis is required for anoikis (anchorage-dependent apoptosis) of non-small cell lung cancer (NSCLC) cells in normal glucose media. Phosphorylation of DAPK at Ser734 by ERK was essential for p53 transcriptional activity and radiosensitization. In HG media, overexpressed DANGER directly bound to the death domain of DAPK, thus inhibiting the catalytic activity of DAPK. In addition, inhibition of the DAPK-p53 signaling axis by DANGER promoted anoikis-resistance and epithelial-mesenchymal transition (EMT), resulting in radioresistance of HG-treated NSCLC cells. Notably, knockdown of DANGER enhanced anoikis, EMT inhibition, and radiosensitization in a mouse xenograft model of lung cancer. Taken together, our findings offered evidence that overexpression of DANGER and the subsequent inhibitory effect on DAPK kinase activity are critical responses that account for HG-induced radioresistance of NSCLC.
Keywords: DANGER; DAPK; anoikis; high glucose; radioresistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Rhamnetin and cirsiliol induce radiosensitization and inhibition of epithelial-mesenchymal transition (EMT) by miR-34a-mediated suppression of Notch-1 expression in non-small cell lung cancer cell lines.J Biol Chem. 2013 Sep 20;288(38):27343-27357. doi: 10.1074/jbc.M113.490482. Epub 2013 Jul 31. J Biol Chem. 2013. PMID: 23902763 Free PMC article.
-
Oroxylin A sensitizes non-small cell lung cancer cells to anoikis via glucose-deprivation-like mechanisms: c-Src and hexokinase II.Biochim Biophys Acta. 2013 Jun;1830(6):3835-45. doi: 10.1016/j.bbagen.2013.03.009. Epub 2013 Mar 15. Biochim Biophys Acta. 2013. PMID: 23500080
-
Plasminogen activator inhibitor-1 enhances radioresistance and aggressiveness of non-small cell lung cancer cells.Oncotarget. 2016 Apr 26;7(17):23961-74. doi: 10.18632/oncotarget.8208. Oncotarget. 2016. PMID: 27004408 Free PMC article.
-
DAPK and cytoskeleton-associated functions.Apoptosis. 2014 Feb;19(2):329-38. doi: 10.1007/s10495-013-0916-5. Apoptosis. 2014. PMID: 24166137 Review.
-
Targeting the Hippo pathway to prevent radioresistance brain metastases from the lung (Review).Int J Oncol. 2024 Jul;65(1):68. doi: 10.3892/ijo.2024.5656. Epub 2024 May 24. Int J Oncol. 2024. PMID: 38785155 Free PMC article. Review.
Cited by
-
TFAP2C promotes lung tumorigenesis and aggressiveness through miR-183- and miR-33a-mediated cell cycle regulation.Oncogene. 2017 Mar;36(11):1585-1596. doi: 10.1038/onc.2016.328. Epub 2016 Sep 5. Oncogene. 2017. PMID: 27593936
-
Differential Talin cleavage in transformed and non-transformed cells and its consequences.Front Cell Dev Biol. 2024 Jul 17;12:1430728. doi: 10.3389/fcell.2024.1430728. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39086658 Free PMC article.
-
Anoikis-related signature predicts prognosis and characterizes immune landscape of ovarian cancer.Cancer Cell Int. 2024 Feb 3;24(1):53. doi: 10.1186/s12935-023-03170-8. Cancer Cell Int. 2024. PMID: 38310291 Free PMC article.
-
Targeting EP4 downstream c-Jun through ERK1/2-mediated reduction of DNMT1 reveals novel mechanism of solamargine-inhibited growth of lung cancer cells.J Cell Mol Med. 2017 Feb;21(2):222-233. doi: 10.1111/jcmm.12958. Epub 2016 Sep 13. J Cell Mol Med. 2017. PMID: 27620163 Free PMC article.
-
Anoikis-Associated Lung Cancer Metastasis: Mechanisms and Therapies.Cancers (Basel). 2022 Sep 30;14(19):4791. doi: 10.3390/cancers14194791. Cancers (Basel). 2022. PMID: 36230714 Free PMC article. Review.
References
-
- Koh PK, Faivre-Finn C, Blackhall FH, De Ruysscher D. Targeted agents in non-small cell lung cancer (NSCLC): clinical developments and rationale for the combination with thoracic radiotherapy. Cancer Treat Rev. 2012;38:626–640. - PubMed
-
- Bussink J, van der Kogel AJ, Kaanders JH. Activation of the PI3-K/AKT pathway and implications for radioresistance mechanisms in head and neck cancer. Lancet Oncol. 2008;9:288–296. - PubMed
-
- Nishimura Y, Nakagawa K, Takeda K, Tanaka M, Segawa Y, Tsujino K, Negoro S, Fuwa N, Hida T, Kawahara M, Katakami N, Hirokawa K, Yamamoto N, et al. Phase I/II trial of sequential chemoradiotherapy using a novel hypoxic cell radiosensitizer, doranidazole (PR-350), in patients with locally advanced non-small-cell lung Cancer (WJTOG-0002) Int J Radiat Oncol Biol Phys. 2007;69:786–792. - PubMed
-
- Kim E, Youn H, Kwon T, Son B, Kang J, Yang HJ, Seong KM, Kim W, Youn B. PAK1 tyrosine phosphorylation is required to induce epithelial-mesenchymal transition and radioresistance in lung cancer cells. Cancer Res. 2014;74:5520–5531. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

