Stimulating the Release of Exosomes Increases the Intercellular Transfer of Prions
- PMID: 26769968
- PMCID: PMC4777847
- DOI: 10.1074/jbc.M115.684258
Stimulating the Release of Exosomes Increases the Intercellular Transfer of Prions
Abstract
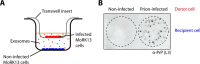
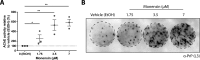
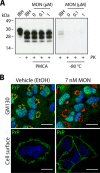
Exosomes are small extracellular vesicles released by cells and play important roles in intercellular communication and pathogen transfer. Exosomes have been implicated in several neurodegenerative diseases, including prion disease and Alzheimer disease. Prion disease arises upon misfolding of the normal cellular prion protein, PrP(C), into the disease-associated isoform, PrP(Sc). The disease has a unique transmissible etiology, and exosomes represent a novel and efficient method for prion transmission. The precise mechanism by which prions are transmitted from cell to cell remains to be fully elucidated, although three hypotheses have been proposed: direct cell-cell contact, tunneling nanotubes, and exosomes. Given the reported presence of exosomes in biological fluids and in the lipid and nucleic acid contents of exosomes, these vesicles represent an ideal mechanism for encapsulating prions and potential cofactors to facilitate prion transmission. This study investigates the relationship between exosome release and intercellular prion dissemination. Stimulation of exosome release through treatment with an ionophore, monensin, revealed a corresponding increase in intercellular transfer of prion infectivity. Conversely, inhibition of exosome release using GW4869 to target the neutral sphingomyelinase pathway induced a decrease in intercellular prion transmission. Further examination of the effect of monensin on PrP conversion revealed that monensin also alters the conformational stability of PrP(C), leading to increased generation of proteinase K-resistant prion protein. The findings presented here provide support for a positive relationship between exosome release and intercellular transfer of prion infectivity, highlighting an integral role for exosomes in facilitating the unique transmissible nature of prions.
Keywords: cell biology; exosome (vesicle); exosomes; extracellular vesicles; monensin; neutral sphingomy; neutral sphingomyelinase; prion; prion disease; transwell.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
Similar articles
-
The neutral sphingomyelinase pathway regulates packaging of the prion protein into exosomes.J Biol Chem. 2015 Feb 6;290(6):3455-67. doi: 10.1074/jbc.M114.605253. Epub 2014 Dec 10. J Biol Chem. 2015. PMID: 25505180 Free PMC article.
-
Autophagy regulates exosomal release of prions in neuronal cells.J Biol Chem. 2018 Jun 8;293(23):8956-8968. doi: 10.1074/jbc.RA117.000713. Epub 2018 Apr 26. J Biol Chem. 2018. PMID: 29700113 Free PMC article.
-
Packaging of prions into exosomes is associated with a novel pathway of PrP processing.J Pathol. 2007 Apr;211(5):582-590. doi: 10.1002/path.2145. J Pathol. 2007. PMID: 17334982
-
Prions and exosomes: from PrPc trafficking to PrPsc propagation.Blood Cells Mol Dis. 2005 Sep-Oct;35(2):143-8. doi: 10.1016/j.bcmd.2005.06.013. Blood Cells Mol Dis. 2005. PMID: 16099696 Review.
-
Exosomes and their role in the intercellular trafficking of normal and disease associated prion proteins.Mol Aspects Med. 2018 Apr;60:62-68. doi: 10.1016/j.mam.2017.11.011. Epub 2017 Dec 8. Mol Aspects Med. 2018. PMID: 29196098 Review.
Cited by
-
Extracellular Vesicles: The Invisible Heroes and Villains of COVID-19 Central Neuropathology.Adv Sci (Weinh). 2024 Mar;11(10):e2305554. doi: 10.1002/advs.202305554. Epub 2023 Dec 24. Adv Sci (Weinh). 2024. PMID: 38143270 Free PMC article. Review.
-
Sialylation of Glycosylphosphatidylinositol (GPI) Anchors of Mammalian Prions Is Regulated in a Host-, Tissue-, and Cell-specific Manner.J Biol Chem. 2016 Aug 12;291(33):17009-19. doi: 10.1074/jbc.M116.732040. Epub 2016 Jun 17. J Biol Chem. 2016. PMID: 27317661 Free PMC article.
-
Cellular mechanisms responsible for cell-to-cell spreading of prions.Cell Mol Life Sci. 2018 Jul;75(14):2557-2574. doi: 10.1007/s00018-018-2823-y. Epub 2018 May 14. Cell Mol Life Sci. 2018. PMID: 29761205 Free PMC article. Review.
-
Exosomes in Parkinson's Disease.Neurosci Bull. 2017 Jun;33(3):331-338. doi: 10.1007/s12264-016-0092-z. Epub 2016 Dec 26. Neurosci Bull. 2017. PMID: 28025780 Free PMC article. Review.
-
Characterization of blood-derived exosomal proteins after exercise.J Int Med Res. 2020 Sep;48(9):300060520957541. doi: 10.1177/0300060520957541. J Int Med Res. 2020. PMID: 32972266 Free PMC article.
References
-
- Zitvogel L., Regnault A., Lozier A., Wolfers J., Flament C., Tenza D., Ricciardi-Castagnoli P., Raposo G., and Amigorena S. (1998) Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat. Med. 4, 594–600 - PubMed
-
- Izquierdo-Useros N., Naranjo-Gómez M., Archer J., Hatch S. C., Erkizia I., Blanco J., Borràs F. E., Puertas M. C., Connor J. H., Fernández-Figueras M. T., Moore L., Clotet B., Gummuluru S., and Martinez-Picado J. (2009) Capture and transfer of HIV-1 particles by mature dendritic cells converges with the exosome-dissemination pathway. Blood 113, 2732–2741 - PMC - PubMed
-
- Caby M. P., Lankar D., Vincendeau-Scherrer C., Raposo G., and Bonnerot C. (2005) Exosomal-like vesicles are present in human blood plasma. Int. Immunol. 17, 879–887 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

