Effect of Periodic Granulocyte Colony-Stimulating Factor Administration on Endothelial Progenitor Cells and Different Monocyte Subsets in Pediatric Patients with Muscular Dystrophies
- PMID: 26770204
- PMCID: PMC4684893
- DOI: 10.1155/2016/2650849
Effect of Periodic Granulocyte Colony-Stimulating Factor Administration on Endothelial Progenitor Cells and Different Monocyte Subsets in Pediatric Patients with Muscular Dystrophies
Abstract
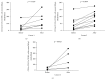
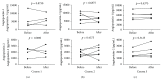
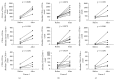
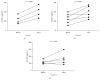
Muscular dystrophies (MD) are heterogeneous group of diseases characterized by progressive muscle dysfunction. There is a large body of evidence indicating that angiogenesis is impaired in muscles of MD patients. Therefore, induction of dystrophic muscle revascularization should become a novel approach aimed at diminishing the extent of myocyte damage. Recently, we and others demonstrated that administration of granulocyte colony-stimulating factor (G-CSF) resulted in clinical improvement of patients with neuromuscular disorders. To date, however, the exact mechanisms underlying these beneficial effects of G-CSF have not been fully understood. Here we used flow cytometry to quantitate numbers of CD34+ cells, endothelial progenitor cells, and different monocyte subsets in peripheral blood of pediatric MD patients treated with repetitive courses of G-CSF administration. We showed that repetitive cycles of G-CSF administration induced efficient mobilization of above-mentioned cells including cells with proangiogenic potential. These findings contribute to better understanding the beneficial clinical effects of G-CSF in pediatric MD patients.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

