Using Computational Modeling To Optimize the Design of Antibodies That Trap Viruses in Mucus
- PMID: 26771004
- PMCID: PMC4707974
- DOI: 10.1021/acsinfecdis.5b00108
Using Computational Modeling To Optimize the Design of Antibodies That Trap Viruses in Mucus
Abstract
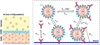
Immunoglobulin G (IgG) antibodies that trap viruses in cervicovaginal mucus (CVM) via adhesive interactions between IgG-Fc and mucins have recently emerged as a promising strategy to block vaginally transmitted infections. The array of IgG bound to a virus particle appears to trap the virus by making multiple weak affinity bonds to the fibrous mucins that form the mucus gel. However, the antibody characteristics that maximize virus trapping and minimize viral infectivity remain poorly understood. Toward this goal, we developed a mathematical model that takes into account physiologically relevant spatial dimensions and time scales, binding, and unbinding rates between IgG and virions and between IgG and mucins, as well as the respective diffusivities of virions and IgG in semen and CVM. We then systematically explored the IgG-antigen and IgG-mucin binding and unbinding rates that minimize the flux of infectious HIV arriving at the vaginal epithelium. Surprisingly, contrary to common intuition that infectivity would drop monotonically with increasing affinities between IgG and HIV, and between IgG and mucins, our model suggests maximal trapping of HIV and minimal flux of HIV to the epithelium are achieved with IgG molecules that exhibit (i) rapid antigen binding (high kon) rather than very slow unbinding (low koff), that is, high-affinity binding to the virion, and (ii) relatively weak affinity with mucins. These results provide important insights into the design of more potent "mucotrapping" IgG for enhanced protection against vaginally transmitted infections. The model is adaptable to other pathogens, mucosal barriers, geometries, and kinetic and diffusional effects, providing a tool for hypothesis testing and producing quantitative insights into the dynamics of immune-mediated protection.
Keywords: HIV; IgG; cervicovaginal mucus; mucin; mucosal immunity; sexually transmitted infections.
Figures
Similar articles
-
The biophysical principles underpinning muco-trapping functions of antibodies.Hum Vaccin Immunother. 2022 Apr 29;18(2):1939605. doi: 10.1080/21645515.2021.1939605. Epub 2021 Jul 27. Hum Vaccin Immunother. 2022. PMID: 34314289 Free PMC article. Review.
-
Broadly neutralizing antibodies consistently trap HIV-1 in fresh cervicovaginal mucus from select individuals.Acta Biomater. 2023 Oct 1;169:387-397. doi: 10.1016/j.actbio.2023.07.031. Epub 2023 Jul 26. Acta Biomater. 2023. PMID: 37499728 Free PMC article.
-
Transient antibody-mucin interactions produce a dynamic molecular shield against viral invasion.Biophys J. 2014 May 6;106(9):2028-36. doi: 10.1016/j.bpj.2014.02.038. Biophys J. 2014. PMID: 24806935 Free PMC article.
-
Modeling Barrier Properties of Intestinal Mucus Reinforced with IgG and Secretory IgA against Motile Bacteria.ACS Infect Dis. 2019 Sep 13;5(9):1570-1580. doi: 10.1021/acsinfecdis.9b00109. Epub 2019 Jul 3. ACS Infect Dis. 2019. PMID: 31268295
-
Tethering of soluble immune effectors to mucin and chitin reflects a convergent and dynamic role in gut immunity.Philos Trans R Soc Lond B Biol Sci. 2024 May 6;379(1901):20230078. doi: 10.1098/rstb.2023.0078. Epub 2024 Mar 18. Philos Trans R Soc Lond B Biol Sci. 2024. PMID: 38497268 Free PMC article. Review.
Cited by
-
Stable nebulization and muco-trapping properties of regdanvimab/IN-006 support its development as a potent, dose-saving inhaled therapy for COVID-19.Bioeng Transl Med. 2022 Aug 30;8(1):e10391. doi: 10.1002/btm2.10391. Online ahead of print. Bioeng Transl Med. 2022. PMID: 36248234 Free PMC article.
-
Diffusion of Immunoglobulin G in Shed Vaginal Epithelial Cells and in Cell-Free Regions of Human Cervicovaginal Mucus.PLoS One. 2016 Jun 30;11(6):e0158338. doi: 10.1371/journal.pone.0158338. eCollection 2016. PLoS One. 2016. PMID: 27362256 Free PMC article.
-
Robust antigen-specific tuning of the nanoscale barrier properties of biogels using matrix-associating IgG and IgM antibodies.Acta Biomater. 2019 Apr 15;89:95-103. doi: 10.1016/j.actbio.2019.03.023. Epub 2019 Mar 14. Acta Biomater. 2019. PMID: 30878451 Free PMC article.
-
Antibody-mediated trapping in biological hydrogels is governed by sugar-sugar hydrogen bonds.Acta Biomater. 2020 Apr 15;107:91-101. doi: 10.1016/j.actbio.2020.03.002. Epub 2020 Mar 5. Acta Biomater. 2020. PMID: 32147470 Free PMC article.
-
The biophysical principles underpinning muco-trapping functions of antibodies.Hum Vaccin Immunother. 2022 Apr 29;18(2):1939605. doi: 10.1080/21645515.2021.1939605. Epub 2021 Jul 27. Hum Vaccin Immunother. 2022. PMID: 34314289 Free PMC article. Review.
References
-
- Casadevall A, Pirofski LA. A new synthesis for antibody-mediated immunity. Nat. Immunol. 2012;13(1):21–28. - PMC - PubMed
- Corthesy B. Role of secretory immunoglobulin A and secretory component in the protection of mucosal surfaces. Future Microbiol. 2010;5(5):817–829. - PubMed
- Kozlowski PA, Neutra MR. The role of mucosal immunity in prevention of HIV transmission. Curr. Mol. Med. 2003;3(3):217–228. - PubMed
-
- Dunkelberger JR, Song WC. Complement and its role in innate and adaptive immune responses. Cell Res. 2010;20(1):34–50. - PubMed
- Huber M, Olson WC, Trkola A. Antibodies for HIV treatment and prevention: window of opportunity? Curr. Top. Microbiol. Immunol. 2008;317:39–66. - PubMed
- Kilian M, Russell MW. Function of mucosal immunoglobulins. In: Ogra PL, Mestecky J, Lamm ME, Strober W, Bienenstock J, McGhee JR, editors. Handbook of Mucosal Immunology. San Diego, CA, USA: Academic Press; 1994. pp. 127–137.
-
- Whaley KJ, Zeitlin L, Barratt RA, Hoen TE, Cone RA. Passive immunization of the vagina protects mice against vaginal transmission of genital herpes infections. J. Infect. Dis. 1994;169(3):647–649. - PubMed
- Zeitlin L, Whaley KJ, Sanna PP, Moench TR, Bastidas R, De Logu A, Williamson RA, Burton DR, Cone RA. Topically applied human recombinant monoclonal IgG1 antibody and its Fab and F(ab′)2 fragments protect mice from vaginal transmission of HSV-2. Virology. 1996;225(1):213–215. - PubMed
- Veazey RS, Shattock RJ, Pope M, Kirijan JC, Jones J, Hu Q, Ketas T, Marx PA, Klasse PJ, Burton DR, Moore JP. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat. Med. 2003;9(3):343–346. - PubMed
- Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, Beary H, Hayes D, Frankel SS, Birx DL, Lewis MG. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 2000;6(2):207–210. - PubMed
-
- Chipperfield EJ, Evans BA. Effect of local infection and oral contraception on immunoglobulin levels in cervical mucus. Infect. Immun. 1975;11(2):215–221. - PMC - PubMed
- Usala SJ, Usala FO, Haciski R, Holt JA, Schumacher GF. IgG and IgA content of vaginal fluid during the menstrual cycle. J. Reprod. Med. 1989;34(4):292–294. - PubMed
- Wang YY, Kannan A, Nunn KL, Murphy MA, Subramani DB, Moench T, Cone R, Lai SK. IgG in cervicovaginal mucus traps HSV and prevents vaginal herpes infections. Mucosal Immunol. 2014;7(5):1036–1044. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

