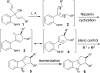
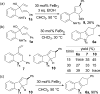
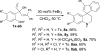Nazarov cyclization of 1,4-pentadien-3-ols: preparation of cyclopenta[b]indoles and spiro[indene-1,4'-quinoline]s
- PMID: 26771024
- PMCID: PMC5560599
- DOI: 10.1039/c5cc08596a
Nazarov cyclization of 1,4-pentadien-3-ols: preparation of cyclopenta[b]indoles and spiro[indene-1,4'-quinoline]s
Abstract
The first Lewis acid-catalyzed intramolecular interrupted Nazarov cyclization of 1,4-pentadien-3-ols is described. Using FeBr3 as the catalyst, a series of new substituted cyclopenta[b]indoles was prepared-through a sequence of Nazarov cyclization, nucleophilic amination, and isomerization-with good yields and high diastereo- and regioselectivities. A similar catalytic process was also developed for the synthesis of structurally interesting spiro[indene-1,4'-quinoline]s.
Figures
References
-
- Kong YC, Cheng KF, Cambie RC, Waterman PG. J Chem Soc, Chem Commun. 1985;47
-
- Stratmann K, Moore RE, Bonjouklian R, Deeter JB, Patterson GML, Shaffer S, Smith CD, Smitka TA. J Am Chem Soc. 1994;116:9935.
-
- Nakazawa J, Yajima J, Usui T, Ueki M, Takatsuki A, Imoto M, Toyoshima YY, Osada H. Chem Biol. 2005;10:131. - PubMed
-
- Jordan JA, Gribble GW, Badenock JC. Tetrahedron Lett. 2011;52:6772.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources