Cytoskeletal Expression and Remodeling in Pluripotent Stem Cells
- PMID: 26771179
- PMCID: PMC4714815
- DOI: 10.1371/journal.pone.0145084
Cytoskeletal Expression and Remodeling in Pluripotent Stem Cells
Abstract
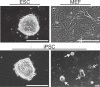
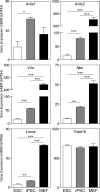
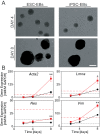
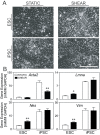
Many emerging cell-based therapies are based on pluripotent stem cells, though complete understanding of the properties of these cells is lacking. In these cells, much is still unknown about the cytoskeletal network, which governs the mechanoresponse. The objective of this study was to determine the cytoskeletal state in undifferentiated pluripotent stem cells and remodeling with differentiation. Mouse embryonic stem cells (ESCs) and reprogrammed induced pluripotent stem cells (iPSCs), as well as the original un-reprogrammed embryonic fibroblasts (MEFs), were evaluated for expression of cytoskeletal markers. We found that pluripotent stem cells overall have a less developed cytoskeleton compared to fibroblasts. Gene and protein expression of smooth muscle cell actin, vimentin, lamin A, and nestin were markedly lower for ESCs than MEFs. Whereas, iPSC samples were heterogeneous with most cells expressing patterns of cytoskeletal proteins similar to ESCs with a small subpopulation similar to MEFs. This indicates that dedifferentiation during reprogramming is associated with cytoskeletal remodeling to a less developed state. In differentiation studies, it was found that shear stress-mediated differentiation resulted in an increase in expression of cytoskeletal intermediate filaments in ESCs, but not in iPSC samples. In the embryoid body model of spontaneous differentiation of pluripotent stem cells, however, both ESCs and iPSCs had similar gene expression for cytoskeletal proteins during early differentiation. With further differentiation, however, gene levels were significantly higher for iPSCs compared to ESCs. These results indicate that reprogrammed iPSCs more readily reacquire cytoskeletal proteins compared to the ESCs that need to form the network de novo. The strategic selection of the parental phenotype is thus critical not only in the context of reprogramming but also the ultimate functionality of the iPSC-differentiated cell population. Overall, this increased characterization of the cytoskeleton in pluripotent stem cells will allow for the better understanding and design of stem cell-based therapies.
Conflict of interest statement
Figures

References
-
- Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676. - PubMed
-
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131: 861–872. - PubMed
-
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science 318: 1917–1920. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

