LEDGF/p75 Overexpression Attenuates Oxidative Stress-Induced Necrosis and Upregulates the Oxidoreductase ERP57/PDIA3/GRP58 in Prostate Cancer
- PMID: 26771192
- PMCID: PMC4714844
- DOI: 10.1371/journal.pone.0146549
LEDGF/p75 Overexpression Attenuates Oxidative Stress-Induced Necrosis and Upregulates the Oxidoreductase ERP57/PDIA3/GRP58 in Prostate Cancer
Abstract
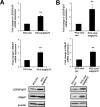
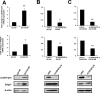
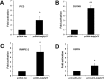
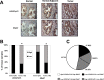
Prostate cancer (PCa) mortality is driven by highly aggressive tumors characterized by metastasis and resistance to therapy, and this aggressiveness is mediated by numerous factors, including activation of stress survival pathways in the pro-inflammatory tumor microenvironment. LEDGF/p75, also known as the DFS70 autoantigen, is a stress transcription co-activator implicated in cancer, HIV-AIDS, and autoimmunity. This protein is targeted by autoantibodies in certain subsets of patients with PCa and inflammatory conditions, as well as in some apparently healthy individuals. LEDGF/p75 is overexpressed in PCa and other cancers, and promotes resistance to chemotherapy-induced cell death via the transactivation of survival proteins. We report in this study that overexpression of LEDGF/p75 in PCa cells attenuates oxidative stress-induced necrosis but not staurosporine-induced apoptosis. This finding was consistent with the observation that while LEDGF/p75 was robustly cleaved in apoptotic cells into a p65 fragment that lacks stress survival activity, it remained relatively intact in necrotic cells. Overexpression of LEDGF/p75 in PCa cells led to the upregulation of transcript and protein levels of the thiol-oxidoreductase ERp57 (also known as GRP58 and PDIA3), whereas its depletion led to ERp57 transcript downregulation. Chromatin immunoprecipitation and transcription reporter assays showed LEDGF/p75 binding to and transactivating the ERp57 promoter, respectively. Immunohistochemical analysis revealed significantly elevated co-expression of these two proteins in clinical prostate tumor tissues. Our results suggest that LEDGF/p75 is not an inhibitor of apoptosis but rather an antagonist of oxidative stress-induced necrosis, and that its overexpression in PCa leads to ERp57 upregulation. These findings are of significance in clarifying the role of the LEDGF/p75 stress survival pathway in PCa.
Conflict of interest statement
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

