Differentiation of Human Induced-Pluripotent Stem Cells into Smooth-Muscle Cells: Two Novel Protocols
- PMID: 26771193
- PMCID: PMC4714916
- DOI: 10.1371/journal.pone.0147155
Differentiation of Human Induced-Pluripotent Stem Cells into Smooth-Muscle Cells: Two Novel Protocols
Abstract
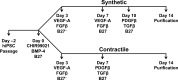
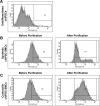
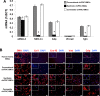
Conventional protocols for differentiating human induced-pluripotent stem cells (hiPSCs) into smooth-muscle cells (SMCs) can be inefficient and generally fail to yield cells with a specific SMC phenotype (i.e., contractile or synthetic SMCs). Here, we present two novel hiPSC-SMC differentiation protocols that yield SMCs with predominantly contractile or synthetic phenotypes. Flow cytometry analyses of smooth-muscle actin (SMA) expression indicated that ~45% of the cells obtained with each protocol assumed an SMC phenotype, and that the populations could be purified to ~95% via metabolic selection. Assessments of cellular mRNA and/or protein levels indicated that SMA, myosin heavy chain II, collagen 1, calponin, transgelin, connexin 43, and vimentin expression in the SMCs obtained via the Contractile SMC protocol and in SMCs differentiated via a traditional protocol were similar, while SMCs produced via the Sythetic SMC protocol expressed less calponin, more collagen 1, and more connexin 43. Differences were also observed in functional assessments of the two SMC populations: the two-dimensional surface area of Contractile SMCs declined more extensively (to 12% versus 44% of original size) in response to carbachol treatment, while quantification of cell migration and proliferation were greater in Synthetic SMCs. Collectively, these data demonstrate that our novel differentiation protocols can efficiently generate SMCs from hiPSCs.
Conflict of interest statement
Figures

References
-
- Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cardiac neural crest. Development 2000;127(8):1607–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

