Preparation of a β-Cyclodextrin-Based Open-Tubular Capillary Electrochromatography Column and Application for Enantioseparations of Ten Basic Drugs
- PMID: 26771454
- PMCID: PMC4714747
- DOI: 10.1371/journal.pone.0146292
Preparation of a β-Cyclodextrin-Based Open-Tubular Capillary Electrochromatography Column and Application for Enantioseparations of Ten Basic Drugs
Abstract
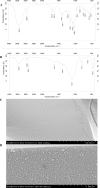
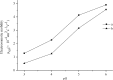
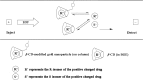
An open-tubular capillary electrochromatography column was prepared by chemically immobilized β-cyclodextrin modified gold nanoparticles onto new surface with the prederivatization of (3-mercaptopropyl)-trimethoxysilane. The synthesized nanoparticles and the prepared column were characterized by transmission electron microscopy, scanning electron microscopy, infrared spectroscopy and ultraviolet visible spectroscopy. When the column was employed as the chiral stationary phase, no enantioselectivity was observed for ten model basic drugs. So β-cyclodextrin was added to the background electrolyte as chiral additive to expect a possible synergistic effect occurring and resulting in a better separation. Fortunately, significant improvement in enantioselectivity was obtained for ten pairs of drug enantiomers. Then, the effects of β-cyclodextrin concentration and background electrolyte pH on the chiral separation were investigated. With the developed separation mode, all the enantiomers (except for venlafaxine) were baseline separated in resolutions of 4.49, 1.68, 1.88, 1.57, 2.52, 2.33, 3.24, 1.63 and 3.90 for zopiclone, chlorphenamine maleate, brompheniramine maleate, dioxopromethazine hydrochloride, carvedilol, homatropine hydrobromide, homatropine methylbromide, venlafaxine, sibutramine hydrochloride and terbutaline sulfate, respectively. Further, the possible separation mechanism involved was discussed.
Conflict of interest statement
Figures




Similar articles
-
Preparation of a thiols β-cyclodextrin/gold nanoparticles-coated open tubular column for capillary electrochromatography enantioseparations.J Sep Sci. 2020 Jun;43(11):2209-2216. doi: 10.1002/jssc.201901323. Epub 2020 Apr 13. J Sep Sci. 2020. PMID: 32160391
-
A novel open-tubular capillary electrochromatography using carboxymethyl-β-cyclodextrin functionalized gold nanoparticles as chiral stationary phase.J Sep Sci. 2020 Mar;43(5):946-953. doi: 10.1002/jssc.201900733. Epub 2019 Dec 27. J Sep Sci. 2020. PMID: 31802622
-
Capillary electrophoretic enantioseparation of basic drugs using a new single-isomer cyclodextrin derivative and theoretical study of the chiral recognition mechanism.J Sep Sci. 2016 May;39(9):1766-75. doi: 10.1002/jssc.201501026. Epub 2016 Apr 5. J Sep Sci. 2016. PMID: 26935589
-
Separation of enantiomers by open capillary electrochromatography on polysiloxane-bonded permethyl-beta-cyclodextrin.J Biochem Biophys Methods. 2001 Apr 24;48(2):117-41. doi: 10.1016/s0165-022x(01)00144-0. J Biochem Biophys Methods. 2001. PMID: 11356483 Review.
-
Recent innovations in enantiomer separation by electrochromatography utilizing modified cyclodextrins as stationary phases.Electrophoresis. 1999 Sep;20(12):2313-28. doi: 10.1002/(SICI)1522-2683(19990801)20:12<2313::AID-ELPS2313>3.0.CO;2-F. Electrophoresis. 1999. PMID: 10499321 Review.
Cited by
-
Gold nanoparticles stabilized with βcyclodextrin-2-amino-4-(4-chlorophenyl)thiazole complex: A novel system for drug transport.PLoS One. 2017 Oct 11;12(10):e0185652. doi: 10.1371/journal.pone.0185652. eCollection 2017. PLoS One. 2017. PMID: 29020065 Free PMC article.
-
Gold nanoparticles coated with a tetramethylammonium lactobionate ionic liquid for enhanced chiral differentiation in open tubular capillary electrochromatography: application to enantioseparation of β-blockers.Mikrochim Acta. 2020 Feb 14;187(3):170. doi: 10.1007/s00604-020-4121-2. Mikrochim Acta. 2020. PMID: 32060642
-
Interpol review of controlled substances 2016-2019.Forensic Sci Int Synerg. 2020 May 24;2:608-669. doi: 10.1016/j.fsisyn.2020.01.019. eCollection 2020. Forensic Sci Int Synerg. 2020. PMID: 33385148 Free PMC article. Review.
References
-
- Ammazzalorso A, A Moroso R, Bettoni G, Chiarini M, Filippis BD, Fantacuzzi M, et al. Enantiomeric separation of gemfi brozil chiral analogues by capillary electrophoresis with heptakis(2,3,6-tri-O-methyl)-β-cyclodextrin as chiral selector. Journal Chromatogr A. 2005; 1088:110–120. - PubMed
-
- Mišl’anová C, Hutta M. Role of biological matrices during the analysis of chiral drugs by liquid chromatography. Journal Chromatogr B. 2003; 797: 91–109. - PubMed
-
- Wille K, De Brabander HF, DeWulf E, Van Caeter P, Janssen CR, Vanhaecke L. Coupled chromatographic and mass-spectrometric techniques for the analysis of emerging pollutants in the aquatic environment. Trac-Trend Anal Chem. 2012; 35: 87–108.
-
- Mathys K, Brenneisen R. Determination of (S)-(-)-cathinone and its metabolites (R,S)-(-)-norephedrine and (R,R)-(-)-norpseudoephedrine in urine by high-performance liquid chromatography with photodiode-array detection. J Chromatogr. A. 1992; 593: 79–85. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

