The Protease Ste24 Clears Clogged Translocons
- PMID: 26771486
- PMCID: PMC4715265
- DOI: 10.1016/j.cell.2015.11.053
The Protease Ste24 Clears Clogged Translocons
Abstract
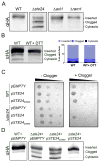
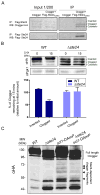
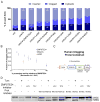
Translocation into the endoplasmic reticulum (ER) is the first step in the biogenesis of thousands of eukaryotic endomembrane proteins. Although functional ER translocation has been avidly studied, little is known about the quality control mechanisms that resolve faulty translocational states. One such faulty state is translocon clogging, in which the substrate fails to properly translocate and obstructs the translocon pore. To shed light on the machinery required to resolve clogging, we carried out a systematic screen in Saccharomyces cerevisiae that highlighted a role for the ER metalloprotease Ste24. We could demonstrate that Ste24 approaches the translocon upon clogging, and it interacts with and generates cleavage fragments of the clogged protein. Importantly, these functions are conserved in the human homolog, ZMPSTE24, although disease-associated mutant forms of ZMPSTE24 fail to clear the translocon. These results shed light on a new and critical task of Ste24, which safeguards the essential process of translocation.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
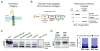



Similar articles
-
Endoplasmic reticulum stress differentially inhibits endoplasmic reticulum and inner nuclear membrane protein quality control degradation pathways.J Biol Chem. 2019 Dec 20;294(51):19814-19830. doi: 10.1074/jbc.RA119.010295. Epub 2019 Nov 13. J Biol Chem. 2019. PMID: 31723032 Free PMC article.
-
Overlapping function of Hrd1 and Ste24 in translocon quality control provides robust channel surveillance.J Biol Chem. 2020 Nov 20;295(47):16113-16120. doi: 10.1074/jbc.AC120.016191. Epub 2020 Oct 8. J Biol Chem. 2020. PMID: 33033070 Free PMC article.
-
The ER-associated protease Ste24 prevents N-terminal signal peptide-independent translocation into the endoplasmic reticulum in Saccharomyces cerevisiae.J Biol Chem. 2020 Jul 24;295(30):10406-10419. doi: 10.1074/jbc.RA120.012575. Epub 2020 Jun 8. J Biol Chem. 2020. PMID: 32513868 Free PMC article.
-
Assembly strategies and GTPase regulation of the eukaryotic and Escherichia coil translocons.Biochem Cell Biol. 2001;79(5):593-601. Biochem Cell Biol. 2001. PMID: 11716301 Review.
-
Ste24: An Integral Membrane Protein Zinc Metalloprotease with Provocative Structure and Emergent Biology.J Mol Biol. 2020 Aug 21;432(18):5079-5090. doi: 10.1016/j.jmb.2020.03.016. Epub 2020 Mar 19. J Mol Biol. 2020. PMID: 32199981 Free PMC article. Review.
Cited by
-
Human CaaX protease ZMPSTE24 expressed in yeast: Structure and inhibition by HIV protease inhibitors.Protein Sci. 2017 Feb;26(2):242-257. doi: 10.1002/pro.3074. Epub 2016 Nov 11. Protein Sci. 2017. PMID: 27774687 Free PMC article.
-
Evaluating protein prenylation of human and viral CaaX sequences using a humanized yeast system.Dis Model Mech. 2024 May 1;17(5):dmm050516. doi: 10.1242/dmm.050516. Epub 2024 May 31. Dis Model Mech. 2024. PMID: 38818856 Free PMC article.
-
Maya Schuldiner: The systems that define us.J Cell Biol. 2016 Apr 11;213(1):3-4. doi: 10.1083/jcb.2131pi. J Cell Biol. 2016. PMID: 27069019 Free PMC article.
-
The Plastoglobule-Localized Metallopeptidase PGM48 Is a Positive Regulator of Senescence in Arabidopsis thaliana.Plant Cell. 2016 Dec;28(12):3020-3037. doi: 10.1105/tpc.16.00745. Epub 2016 Nov 28. Plant Cell. 2016. PMID: 27895226 Free PMC article.
-
ZMPSTE24 defends against influenza and other pathogenic viruses.J Exp Med. 2017 Apr 3;214(4):919-929. doi: 10.1084/jem.20161270. Epub 2017 Feb 28. J Exp Med. 2017. PMID: 28246125 Free PMC article.
References
-
- Ast T, Aviram N, Chuartzman SG, Schuldiner M. A cytosolic degradation pathway, prERAD, monitors pre-inserted secretory pathway proteins. J Cell Sci. 2014;127:3017–3023. - PubMed
-
- Ast T, Cohen G, Schuldiner M. A Network of Cytosolic Factors Targets SRP-Indepenent Proteins to the Endoplasmic Reticulum. Cell. 2013;152:1134–1145. - PubMed
-
- Ast T, Schuldiner M. All roads lead to Rome (but some may be harder to travel): SRP-independent translocation into the endoplasmic reticulum. Crit Rev Biochem Mol Biol. 2013;48:273–288. - PubMed
-
- Avci D, Fuchs S, Schrul B, Fukumori A, Breker M, Frumkin I, Chen CY, Biniossek ML, Kremmer E, Schilling O, et al. The yeast ER-intramembrane protease Ypf1 refines nutrient sensing by regulating transporter abundance. Mol Cell. 2014;56:630–640. - PubMed
-
- Aviram N, Schuldiner M. Embracing the void--how much do we really know about targeting and translocation to the endoplasmic reticulum? Curr Opin Cell Biol. 2014;29:8–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

