Astrocytes Assemble Thalamocortical Synapses by Bridging NRX1α and NL1 via Hevin
- PMID: 26771491
- PMCID: PMC4715262
- DOI: 10.1016/j.cell.2015.11.034
Astrocytes Assemble Thalamocortical Synapses by Bridging NRX1α and NL1 via Hevin
Abstract
Proper establishment of synapses is critical for constructing functional circuits. Interactions between presynaptic neurexins and postsynaptic neuroligins coordinate the formation of synaptic adhesions. An isoform code determines the direct interactions of neurexins and neuroligins across the synapse. However, whether extracellular linker proteins can expand such a code is unknown. Using a combination of in vitro and in vivo approaches, we found that hevin, an astrocyte-secreted synaptogenic protein, assembles glutamatergic synapses by bridging neurexin-1alpha and neuroligin-1B, two isoforms that do not interact with each other. Bridging of neurexin-1alpha and neuroligin-1B via hevin is critical for the formation and plasticity of thalamocortical connections in the developing visual cortex. These results show that astrocytes promote the formation of synapses by modulating neurexin/neuroligin adhesions through hevin secretion. Our findings also provide an important mechanistic insight into how mutations in these genes may lead to circuit dysfunction in diseases such as autism.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
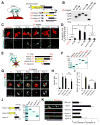

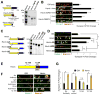
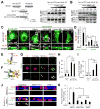
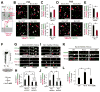

Comment in
-
Synaptogenesis: A synaptic bridge.Nat Rev Neurosci. 2016 Mar;17(3):135. doi: 10.1038/nrn.2016.12. Epub 2016 Feb 11. Nat Rev Neurosci. 2016. PMID: 26865017 No abstract available.
References
-
- Baudouin S, Scheiffele P. SnapShot: Neuroligin-neurexin complexes. Cell. 2010;141:908, 908 e901. - PubMed
-
- Boucard AA, Chubykin AA, Comoletti D, Taylor P, Sudhof TC. A splice code for trans-synaptic cell adhesion mediated by binding of neuroligin 1 to alpha- and beta-neurexins. Neuron. 2005;48:229–236. - PubMed
-
- Bourne HR, Nicoll R. Molecular machines integrate coincident synaptic signals. Cell. 1993;72(Suppl):65–75. - PubMed
-
- Budreck EC, Kwon OB, Jung JH, Baudouin S, Thommen A, Kim HS, Fukazawa Y, Harada H, Tabuchi K, Shigemoto R, et al. Neuroligin-1 controls synaptic abundance of NMDA-type glutamate receptors through extracellular coupling. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:725–730. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DE022743/DE/NIDCR NIH HHS/United States
- T32 HD040372/HD/NICHD NIH HHS/United States
- T32 GM007184/GM/NIGMS NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R01 DE017794/DE/NIDCR NIH HHS/United States
- DA031833/DA/NIDA NIH HHS/United States
- MH103374/MH/NIMH NIH HHS/United States
- R01 AA013023/AA/NIAAA NIH HHS/United States
- NS092419/NS/NINDS NIH HHS/United States
- R01 AA022455/AA/NIAAA NIH HHS/United States
- F31 NS092419/NS/NINDS NIH HHS/United States
- R01 NS083897/NS/NINDS NIH HHS/United States
- DE22743/DE/NIDCR NIH HHS/United States
- AA0130123/AA/NIAAA NIH HHS/United States
- NS083897/NS/NINDS NIH HHS/United States
- R01 DA031833/DA/NIDA NIH HHS/United States
- R01 MH103374/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

