Chemical Structure-Related Drug-Like Criteria of Global Approved Drugs
- PMID: 26771590
- PMCID: PMC6273477
- DOI: 10.3390/molecules21010075
Chemical Structure-Related Drug-Like Criteria of Global Approved Drugs
Abstract
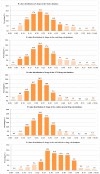
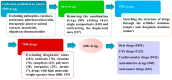
The chemical structure of a drug determines its physicochemical properties, further determines its ADME/Tox properties, and ultimately affects its pharmacological activity. Medicinal chemists can regulate the pharmacological activity of drug molecules by modifying their structure. Ring systems and functional groups are important components of a drug. The proportion of non-hydrocarbon atoms among non-hydrogen atoms reflects the heavy atoms proportion of a drug. The three factors have considerable potential for the assessment of the drug-like properties of organic molecules. However, to the best of our knowledge, there have been no studies to systematically analyze the simultaneous effects of the number of aromatic and non-aromatic rings, the number of some special functional groups and the proportion of heavy atoms on the drug-like properties of an organic molecule. To this end, the numbers of aromatic and non-aromatic rings, the numbers of some special functional groups and the heavy atoms proportion of 6891 global approved small drugs have been comprehensively analyzed. We first uncovered three important structure-related criteria closely related to drug-likeness, namely: (1) the best numbers of aromatic and non-aromatic rings are 2 and 1, respectively; (2) the best functional groups of candidate drugs are usually -OH, -COOR and -COOH in turn, but not -CONHOH, -SH, -CHO and -SO3H. In addition, the -F functional group is beneficial to CNS drugs, and -NH2 functional group is beneficial to anti-infective drugs and anti-cancer drugs; (3) the best R value intervals of candidate drugs are in the range of 0.05-0.50 (preferably 0.10-0.35), and R value of the candidate CNS drugs should be as small as possible in this interval. We envision that the three chemical structure-related criteria may be applicable in a prospective manner for the identification of novel candidate drugs and will provide a theoretical foundation for designing new chemical entities with good drug-like properties.
Keywords: chemical structure-related criteria; drug design; drug-like property; functional group; ring system; the heavy atoms proportion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Price Waterhouse Coopers (PWC) From Vision to Decision. Pharma 2020 Report. PWC; London, UK: 2012. [(accessed on 16 November 2015)]. Available online: http://www.pwc.com/gx/en/industries/pharmaceuticals-life-sciences/pharma....
-
- Borchardt R.T. Pharmaceutical Profiling in Drug Discovery for Lead Selection. American Association of Pharmaceutical Scientists; Arlington, VA, USA: 2004.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

