Overexpression of PRL7D1 in Leydig Cells Causes Male Reproductive Dysfunction in Mice
- PMID: 26771609
- PMCID: PMC4730338
- DOI: 10.3390/ijms17010096
Overexpression of PRL7D1 in Leydig Cells Causes Male Reproductive Dysfunction in Mice
Abstract
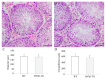
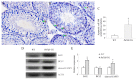
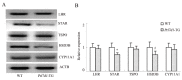
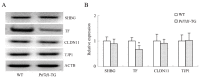
Prolactin family 7, subfamily d, member 1 (PRL7D1) is found in mouse placenta. Our recent work showed that PRL7D1 is also present in mouse testis Leydig cells, and the expression of PRL7D1 in the testis exhibits an age-related increase. In the present study, we generated transgenic mice with Leydig cell-specific PRL7D1 overexpression to explore its function during male reproduction. Prl7d1 male mice exhibited subfertility as reflected by reduced sperm counts and litter sizes. The testes from Prl7d1 transgenic mice appeared histologically normal, but the frequency of apoptotic germ cells was increased. Prl7d1 transgenic mice also had lower testosterone concentrations than wild-type mice. Mechanistic studies revealed that Prl7d1 transgenic mice have defects in the testicular expression of steroidogenic acute regulatory protein (STAR) and hydroxy-delta-5-steroid dehydrogenase, 3 beta- and steroid delta-isomerase cluster (HSD3B). Further studies revealed that PRL7D1 overexpression affected the expression of transferrin (TF) in Sertoli cells. These results suggest that PRL7D1 overexpression could lead to increased germ cell apoptosis and exert an inhibitory effect on testosterone production in Leydig cells by reducing the expression of certain steroidogenic-related genes. In addition, PRL7D1 appears to have important roles in the function of Sertoli cells, which, in turn, affects male fertility. We conclude that the expression level of PRL7D1 is associated with the reproductive function of male mice.
Keywords: Leydig cells; Plr7d1; male reproductive dysfunction.
Figures

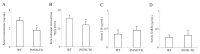
Similar articles
-
Wt1 is involved in leydig cell steroid hormone biosynthesis by regulating paracrine factor expression in mice.Biol Reprod. 2014 Apr 3;90(4):71. doi: 10.1095/biolreprod.113.114702. Print 2014 Apr. Biol Reprod. 2014. PMID: 24571983
-
Fertility and spermatogenesis are altered in {alpha}1b-adrenergic receptor knockout male mice.J Endocrinol. 2007 Nov;195(2):281-92. doi: 10.1677/JOE-07-0071. J Endocrinol. 2007. PMID: 17951539
-
Leydig cell-specific expression of DAX1 improves fertility of the Dax1-deficient mouse.Biol Reprod. 2003 Jul;69(1):154-60. doi: 10.1095/biolreprod.102.011429. Epub 2003 Feb 19. Biol Reprod. 2003. PMID: 12606353
-
Stress hormone and male reproductive function.Cell Tissue Res. 2005 Oct;322(1):147-53. doi: 10.1007/s00441-005-0006-2. Epub 2005 Nov 3. Cell Tissue Res. 2005. PMID: 16079965 Review.
-
The effect of age on male reproductive function.World J Urol. 1993;11(2):137-40. doi: 10.1007/BF00182041. World J Urol. 1993. PMID: 8343797 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

