Purified and Recombinant Hemopexin: Protease Activity and Effect on Neutrophil Chemotaxis
- PMID: 26772775
- PMCID: PMC5004720
- DOI: 10.2119/molmed.2016.00006
Purified and Recombinant Hemopexin: Protease Activity and Effect on Neutrophil Chemotaxis
Abstract
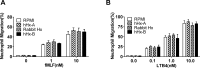
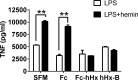
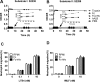
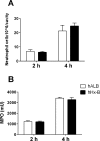
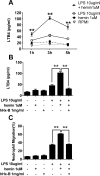
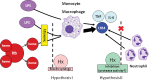
Infusion of the heme-binding protein hemopexin has been proposed as a novel approach to decrease heme-induced inflammation in settings of red blood cell breakdown, but questions have been raised as to possible side effects related to protease activity and inhibition of chemotaxis. We evaluated protease activity and effects on chemotaxis of purified plasma hemopexin obtained from multiple sources as well as a novel recombinant fusion protein Fc-hemopexin. Amidolytic assay was performed to measure the protease activity of several plasma-derived hemopexin and recombinant Fc-hemopexin. Hemopexin was added to the human monocyte culture in the presence of lipopolysaccharides (LPS), and also injected into mice intravenously (i.v.) 30 min before inducing neutrophil migration via intraperitoneal (i.p.) injection of thioglycolate. Control groups received the same amount of albumin. Protease activity varied widely between hemopexins. Recombinant Fc-hemopexin bound heme, inhibited the synergy of heme with LPS on tumor necrosis factor (TNF) production from monocytes, and had minor but detectable protease activity. There was no effect of any hemopexin preparation on chemotaxis, and purified hemopexin did not alter the migration of neutrophils into the peritoneal cavity of mice. Heme and LPS synergistically induced the release of LTB4 from human monocytes, and hemopexin blocked this release, as well as chemotaxis of neutrophils in response to activated monocyte supernatants. These results suggest that hemopexin does not directly affect chemotaxis through protease activity, but may decrease heme-driven chemotaxis and secondary inflammation by attenuating the induction of chemoattractants from monocytes. This property could be beneficial in some settings to control potentially damaging inflammation induced by heme.
Conflict of interest statement
In accordance with institutional policy, HS Warren has declared hemopexin to Massachusetts General Hospital as a potential candidate molecule to help decrease inflammation, and the institution has filed for patent protection. M Super, AL Watters and DE Ingber have filed for patent protection for recombinant Fc-hemopexin.
Figures


Similar articles
-
Growth inhibition of Bacteroides fragilis by hemopexin: proteolytic degradation of hemopexin to overcome heme limitation.FEMS Microbiol Lett. 2001 May 15;199(1):73-8. doi: 10.1111/j.1574-6968.2001.tb10653.x. FEMS Microbiol Lett. 2001. PMID: 11356570
-
Inhibition of neutrophil migration by hemopexin leads to increased mortality due to sepsis in mice.Am J Respir Crit Care Med. 2011 Apr 1;183(7):922-31. doi: 10.1164/rccm.201002-0223OC. Epub 2010 Oct 22. Am J Respir Crit Care Med. 2011. PMID: 20971829
-
Human Plasma and Recombinant Hemopexins: Heme Binding Revisited.Int J Mol Sci. 2021 Jan 26;22(3):1199. doi: 10.3390/ijms22031199. Int J Mol Sci. 2021. PMID: 33530421 Free PMC article.
-
[Serum hemopexin: suppressive effect on neutrophil functions and prospect of clinical application to autoimmune diseases].Nihon Rinsho. 2004 Mar;62(3):577-86. Nihon Rinsho. 2004. PMID: 15038107 Review. Japanese.
-
An alternative view of the proposed alternative activities of hemopexin.Protein Sci. 2011 May;20(5):791-805. doi: 10.1002/pro.616. Epub 2011 Mar 30. Protein Sci. 2011. PMID: 21404362 Free PMC article. Review.
Cited by
-
Deferoxamine therapy reduces brain hemin accumulation after intracerebral hemorrhage in piglets.Exp Neurol. 2019 Aug;318:244-250. doi: 10.1016/j.expneurol.2019.05.003. Epub 2019 May 10. Exp Neurol. 2019. PMID: 31078524 Free PMC article. Review.
-
Heme as a Target for Therapeutic Interventions.Front Pharmacol. 2017 Apr 4;8:146. doi: 10.3389/fphar.2017.00146. eCollection 2017. Front Pharmacol. 2017. PMID: 28420988 Free PMC article. Review.
-
Phosgene inhalation causes hemolysis and acute lung injury.Toxicol Lett. 2019 Sep 15;312:204-213. doi: 10.1016/j.toxlet.2019.04.019. Epub 2019 Apr 30. Toxicol Lett. 2019. PMID: 31047999 Free PMC article.
References
-
- Hebert PC, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N. Engl. J. Med. 1999;340:409–17. - PubMed
-
- Malone DL, Dunne J, Tracy JK, Putnam AT, Scalea TM, Napolitano LM. Blood transfusion, independent of shock severity, is associated with worse outcome in trauma. J. Trauma. 2003;54:898–905. - PubMed
-
- Lacroix J, et al. Transfusion strategies for patients in pediatric intensive care units. N. Engl. J. Med. 2007;356:1609–19. - PubMed
-
- Reeves BC, Murphy GJ. Increased mortality, morbidity, and cost associated with red blood cell transfusion after cardiac surgery. Curr. Opin. Cardiol. 2008;23:607–12. - PubMed
-
- Adamson JW. New blood, old blood, or no blood? N. Engl. J. Med. 2008;358:1295–6. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

