The effect of antiretroviral therapy on all-cause mortality, generalized to persons diagnosed with HIV in the USA, 2009-11
- PMID: 26772869
- PMCID: PMC5013889
- DOI: 10.1093/ije/dyv352
The effect of antiretroviral therapy on all-cause mortality, generalized to persons diagnosed with HIV in the USA, 2009-11
Erratum in
-
Corrigendum to: The effect of antiretroviral therapy on all-cause mortality, generalized to persons diagnosed with HIV in the USA, 2009-11.Int J Epidemiol. 2021 Jul 9;50(3):1044. doi: 10.1093/ije/dyab102. Int J Epidemiol. 2021. PMID: 33993279 Free PMC article. No abstract available.
Abstract
Background: Although antiretroviral therapy (ART) is known to be protective against HIV-related mortality, the expected magnitude of effect is unclear because existing estimates of the effect of ART may not directly generalize to recently HIV-diagnosed persons.
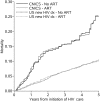
Methods: In this study, we estimated 5-year mortality risks for immediate versus no ART initiation among patients (n = 12,547) in the Centers for AIDS Research Network of Integrated Clinical Systems (CNICS) using the complement of adjusted Kaplan-Meier survival functions. We subsequently standardized estimates to persons diagnosed with HIV in the USA between 2009 and 2011, who were enumerated using national surveillance data.
Results: The 5-year mortality, had all patients in the CNICS immediately initiated ART, was 10.6% [95% confidence interval (CI): 9.3%, 11.9%] compared with 28.3% (95% CI: 19.1%, 37.5%) had ART initiation been delayed at least 5 years. The 5-year mortality risk difference due to ART among patients in the CNICS was -17.7% (95% CI: -27.0%, -8.4%). Based on methods for generalizing an estimate from a study sample to a different target population, the expected risk difference due to ART initiation among recently HIV-diagnosed persons in the USA was -19.1% (95% CI: -30.5%, -7.8%).
Conclusions: Immediate ART initiation substantially lowers mortality among persons in the CNICS and this benefit is expected to be similar among persons recently diagnosed with HIV in the USA. We demonstrate a method by which concerns about generalizability can be addressed and evaluated quantitatively.
Keywords: HIV; antiretroviral therapy; effect modification; external validity; generalizability; mortality; survival analysis.
Published by Oxford University Press on behalf of the International Epidemiological Association 2016. This work is written by US Government employees and is in the public domain in the US.
Figures
References
-
- Hammer SM, Squires KE, Hughes MD, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med 1997;337:725–33. - PubMed
-
- Cameron DW, Heath-Chiozzi M, Danner S, et al. Randomised placebo-controlled trial of ritonavir in advanced HIV-1 disease. The Advanced HIV Disease Ritonavir Study Group. Lancet 1998;351:543–49. - PubMed
-
- Walensky RP, Paltiel AD, Losina E, et al. The survival benefits of AIDS treatment in the USA. J Infect Dis 2006;194:11–19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

