Prrx1 isoform switching regulates pancreatic cancer invasion and metastatic colonization
- PMID: 26773005
- PMCID: PMC4719312
- DOI: 10.1101/gad.263327.115
Prrx1 isoform switching regulates pancreatic cancer invasion and metastatic colonization
Abstract
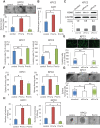
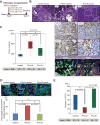
The two major isoforms of the paired-related homeodomain transcription factor 1 (Prrx1), Prrx1a and Prrx1b, are involved in pancreatic development, pancreatitis, and carcinogenesis, although the biological role that these isoforms serve in the systemic dissemination of pancreatic ductal adenocarcinoma (PDAC) has not been investigated. An epithelial-mesenchymal transition (EMT) is believed to be important for primary tumor progression and dissemination, whereas a mesenchymal-epithelial transition (MET) appears crucial for metastatic colonization. Here, we describe novel roles for both isoforms in the metastatic cascade using complementary in vitro and in vivo models. Prrx1b promotes invasion, tumor dedifferentiation, and EMT. In contrast, Prrx1a stimulates metastatic outgrowth in the liver, tumor differentiation, and MET. We further demonstrate that the switch from Prrx1b to Prrx1a governs EMT plasticity in both mouse models of PDAC and human PDAC. Last, we identify hepatocyte growth factor ( HGF) as a novel transcriptional target of Prrx1b. Targeted therapy of HGF in combination with gemcitabine in a preclinical model of PDAC reduces primary tumor volume and eliminates metastatic disease. Overall, we provide new insights into the isoform-specific roles of Prrx1a and Prrx1b in primary PDAC formation, dissemination, and metastatic colonization, allowing for novel therapeutic strategies targeting EMT plasticity.
Keywords: EMT; MET; Prrx1a; Prrx1b; metastasis; pancreatic cancer.
© 2016 Takano et al.; Published by Cold Spring Harbor Laboratory Press.
Figures







Similar articles
-
PRRX1 isoforms cooperate with FOXM1 to regulate the DNA damage response in pancreatic cancer cells.Oncogene. 2019 May;38(22):4325-4339. doi: 10.1038/s41388-019-0725-6. Epub 2019 Jan 31. Oncogene. 2019. PMID: 30705403 Free PMC article.
-
The Prrx1 homeodomain transcription factor plays a central role in pancreatic regeneration and carcinogenesis.Genes Dev. 2013 Feb 1;27(3):288-300. doi: 10.1101/gad.204453.112. Epub 2013 Jan 25. Genes Dev. 2013. PMID: 23355395 Free PMC article.
-
PRRX1 isoform PRRX1A regulates the stemness phenotype and epithelial-mesenchymal transition (EMT) of cancer stem-like cells (CSCs) derived from non-small cell lung cancer (NSCLC).Transl Lung Cancer Res. 2020 Jun;9(3):731-744. doi: 10.21037/tlcr-20-633. Transl Lung Cancer Res. 2020. PMID: 32676335 Free PMC article.
-
Never let it go: Stopping key mechanisms underlying metastasis to fight pancreatic cancer.Semin Cancer Biol. 2017 Jun;44:43-59. doi: 10.1016/j.semcancer.2017.04.006. Epub 2017 Apr 22. Semin Cancer Biol. 2017. PMID: 28438662 Review.
-
Mechanisms Underlying Metastatic Pancreatic Cancer.Adv Exp Med Biol. 2019;1164:3-10. doi: 10.1007/978-3-030-22254-3_1. Adv Exp Med Biol. 2019. PMID: 31576536 Review.
Cited by
-
EMT, MET, Plasticity, and Tumor Metastasis.Trends Cell Biol. 2020 Oct;30(10):764-776. doi: 10.1016/j.tcb.2020.07.003. Epub 2020 Aug 13. Trends Cell Biol. 2020. PMID: 32800658 Free PMC article. Review.
-
The Quasimesenchymal Pancreatic Ductal Epithelial Cell Line PANC-1-A Useful Model to Study Clonal Heterogeneity and EMT Subtype Shifting.Cancers (Basel). 2022 Apr 19;14(9):2057. doi: 10.3390/cancers14092057. Cancers (Basel). 2022. PMID: 35565186 Free PMC article.
-
The Epithelial to Mesenchymal Transition in Colorectal Cancer Progression: The Emerging Role of Succinate Dehydrogenase Alterations and Succinate Accumulation.Biomedicines. 2023 May 11;11(5):1428. doi: 10.3390/biomedicines11051428. Biomedicines. 2023. PMID: 37239099 Free PMC article. Review.
-
PRRX1 promotes malignant properties in human osteosarcoma.Transl Oncol. 2021 Jan;14(1):100960. doi: 10.1016/j.tranon.2020.100960. Epub 2020 Dec 9. Transl Oncol. 2021. PMID: 33395745 Free PMC article.
-
A cellular hierarchy in melanoma uncouples growth and metastasis.Nature. 2022 Oct;610(7930):190-198. doi: 10.1038/s41586-022-05242-7. Epub 2022 Sep 21. Nature. 2022. PMID: 36131018 Free PMC article.
References
-
- Bilimoria KY, Bentrem DJ, Ko CY, Tomlinson JS, Stewart AK, Winchester DP, Talamonti MS. 2007. Multimodality therapy for pancreatic cancer in the U.S.: utilization, outcomes, and the effect of hospital volume. Cancer 110: 1227–1234. - PubMed
-
- Brabletz T. 2012. To differentiate or not—routes towards metastasis. Nat Rev Cancer 12: 425–436. - PubMed
-
- Cardin DB, Berlin JD. 2013. Pancreas cancer on the rise: are we up to the challenge? J Natl Cancer Inst 105: 1675–1676. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
