B-cell survival and development controlled by the coordination of NF-κB family members RelB and cRel
- PMID: 26773039
- PMCID: PMC4786837
- DOI: 10.1182/blood-2014-10-606988
B-cell survival and development controlled by the coordination of NF-κB family members RelB and cRel
Abstract
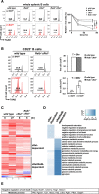
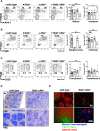
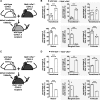
Targeted deletion of BAFF causes severe deficiency of splenic B cells. BAFF-R is commonly thought to signal to nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB)-inducing kinase dependent noncanonical NF-κB RelB. However, RelB-deficient mice have normal B-cell numbers. Recent studies showed that BAFF also signals to the canonical NF-κB pathway, and we found that both RelB and cRel are persistently activated, suggesting BAFF signaling coordinates both pathways to ensure robust B-cell development. Indeed, we report now that combined loss of these 2 NF-κB family members leads to impaired BAFF-mediated survival and development in vitro. Although single deletion of RelB and cRel was dispensable for normal B-cell development, double knockout mice displayed an early B-cell developmental blockade and decreased mature B cells. Despite disorganized splenic architecture in Relb(-/-)cRel(-/-) mice, generation of mixed-mouse chimeras established the developmental phenotype to be B-cell intrinsic. Together, our results indicate that BAFF signals coordinate both RelB and cRel activities to ensure survival during peripheral B-cell maturation.
© 2016 by The American Society of Hematology.
Figures



References
-
- Allman DM, Ferguson SE, Cancro MP. Peripheral B cell maturation. I. Immature peripheral B cells in adults are heat-stable antigenhi and exhibit unique signaling characteristics. J Immunol. 1992;149(8):2533–2540. - PubMed
-
- Allman DM, Ferguson SE, Lentz VM, Cancro MP. Peripheral B cell maturation. II. Heat-stable antigen(hi) splenic B cells are an immature developmental intermediate in the production of long-lived marrow-derived B cells. J Immunol. 1993;151(9):4431–4444. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

