A lymphomagenic role for HIV beyond immune suppression?
- PMID: 26773045
- PMCID: PMC4826146
- DOI: 10.1182/blood-2015-11-681411
A lymphomagenic role for HIV beyond immune suppression?
Abstract
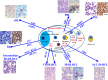
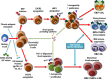
Despite the immune reconstitution promoted by combined antiretroviral therapy (cART), lymphomas still represent the most common type of cancer in HIV-infected individuals. Cofactors related to immunodeficiency such as oncogenic viruses, chronic antigenic stimulation, and cytokine overproduction are thought to be the main drivers of HIV lymphomagenesis, although the current scenario does not convincingly explain the still-high incidence of lymphomas and the occurrence of peculiar lymphoma histotypes in HIV-infected patients under cART. Recent findings are challenging the current view of a mainly indirect role of HIV in lymphoma development and support the possibility that HIV may directly contribute to lymphomagenesis. In fact, mechanisms other than immune suppression involve biologic effects mediated by HIV products that are secreted and accumulate in lymphoid tissues, mainly within lymph node germinal centers. Notably, HIV-infected patients with lymphomas, but not those not affected by these tumors, were recently shown to carry HIV p17 protein variants with enhanced B-cell clonogenic activity. HIV p17 protein variants were characterized by the presence of distinct insertions at the C-terminal region of the protein responsible for a structural destabilization and the acquisition of novel biologic properties. These data are changing the current paradigm assuming that HIV is only indirectly related to lymphomagenesis. Furthermore, these recent findings are consistent with a role of HIV as a critical microenvironmental factor promoting lymphoma development and pave the way for further studies that may lead to the design of more effective strategies for an early identification and improved control of lymphomas in the HIV setting.
© 2016 by The American Society of Hematology.
Figures

References
-
- Carbone A, Vaccher E, Gloghini A, et al. Diagnosis and management of lymphomas and other cancers in HIV-infected patients. Nat Rev Clin Oncol. 2014;11(4):223–238. - PubMed
-
- Grulich AE, Vajdic CM. The epidemiology of cancers in human immunodeficiency virus infection and after organ transplantation. Semin Oncol. 2015;42(2):247–257. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

