Small Heat Shock Proteins Are Novel Common Determinants of Alcohol and Nicotine Sensitivity in Caenorhabditis elegans
- PMID: 26773049
- PMCID: PMC4788107
- DOI: 10.1534/genetics.115.185025
Small Heat Shock Proteins Are Novel Common Determinants of Alcohol and Nicotine Sensitivity in Caenorhabditis elegans
Abstract
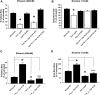
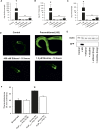
Addiction to drugs is strongly determined by multiple genetic factors. Alcohol and nicotine produce distinct pharmacological effects within the nervous system through discrete molecular targets; yet, data from family and twin analyses support the existence of common genetic factors for addiction in general. The mechanisms underlying addiction, however, are poorly described and common genetic factors for alcohol and nicotine remain unidentified. We investigated the role that the heat shock transcription factor, HSF-1, and its downstream effectors played as common genetic modulators of sensitivity to addictive substances. Using Caenorhabditis elegans, an exemplary model organism with substance dose-dependent responses similar to mammals, we demonstrate that HSF-1 altered sensitivity to both alcohol and nicotine. Using a combination of a targeted RNAi screen of downstream factors and transgenic approaches we identified that these effects were contingent upon the constitutive neuronal expression of HSP-16.48, a small heat shock protein (HSP) homolog of human α-crystallin. Furthermore we demonstrated that the function of HSP-16.48 in drug sensitivity surprisingly was independent of chaperone activity during the heat shock stress response. Instead we identified a distinct domain within the N-terminal region of the HSP-16.48 protein that specified its function in comparison to related small HSPs. Our findings establish and characterize a novel genetic determinant underlying sensitivity to diverse addictive substances.
Keywords: HSF1; alcohol; alpha crystallin; heat shock proteins; nicotine.
Copyright © 2016 by the Genetics Society of America.
Figures




Similar articles
-
Ethanol Stimulates Locomotion via a Gαs-Signaling Pathway in IL2 Neurons in Caenorhabditis elegans.Genetics. 2017 Nov;207(3):1023-1039. doi: 10.1534/genetics.117.300119. Epub 2017 Sep 26. Genetics. 2017. PMID: 28951527 Free PMC article.
-
The genome-wide role of HSF-1 in the regulation of gene expression in Caenorhabditis elegans.BMC Genomics. 2016 Aug 5;17:559. doi: 10.1186/s12864-016-2837-5. BMC Genomics. 2016. PMID: 27496166 Free PMC article.
-
Effects of chlorogenic acid on thermal stress tolerance in C. elegans via HIF-1, HSF-1 and autophagy.Phytomedicine. 2020 Jan;66:153132. doi: 10.1016/j.phymed.2019.153132. Epub 2019 Nov 4. Phytomedicine. 2020. PMID: 31790899
-
The Thermal Stress Coping Network of the Nematode Caenorhabditis elegans.Int J Mol Sci. 2022 Nov 28;23(23):14907. doi: 10.3390/ijms232314907. Int J Mol Sci. 2022. PMID: 36499234 Free PMC article. Review.
-
Transcriptional regulation of small HSP-HSF1 and beyond.Int J Biochem Cell Biol. 2012 Oct;44(10):1593-612. doi: 10.1016/j.biocel.2012.06.012. Epub 2012 Jun 29. Int J Biochem Cell Biol. 2012. PMID: 22750029 Review.
Cited by
-
Evidence for involvement of the alcohol consumption WDPCP gene in lipid metabolism, and liver cirrhosis.Sci Rep. 2023 Nov 23;13(1):20616. doi: 10.1038/s41598-023-47371-7. Sci Rep. 2023. PMID: 37996473 Free PMC article.
-
Functional validity, role, and implications of heavy alcohol consumption genetic loci.Sci Adv. 2020 Jan 15;6(3):eaay5034. doi: 10.1126/sciadv.aay5034. eCollection 2020 Jan. Sci Adv. 2020. PMID: 31998841 Free PMC article.
-
BK channels and alcohol tolerance: Insights from studies on Drosophila, nematodes, rodents and cell lines: A systematic review.Med Int (Lond). 2025 Apr 2;5(4):33. doi: 10.3892/mi.2025.232. eCollection 2025 Jul-Aug. Med Int (Lond). 2025. PMID: 40236633 Free PMC article.
-
FNDC-1-mediated mitophagy and ATFS-1 coordinate to protect against hypoxia-reoxygenation.Autophagy. 2021 Nov;17(11):3389-3401. doi: 10.1080/15548627.2021.1872885. Epub 2021 Jan 19. Autophagy. 2021. PMID: 33416042 Free PMC article.
-
C. elegans dkf-1 (Protein Kinase D1) mutants have age-dependent defects in locomotion and neuromuscular transmission.MicroPubl Biol. 2023 Apr 4;2023:10.17912/micropub.biology.000800. doi: 10.17912/micropub.biology.000800. eCollection 2023. MicroPubl Biol. 2023. PMID: 37090152 Free PMC article.
References
-
- Anckar J., Sistonen L., 2011. Regulation of HSF1 function in the heat stress response: implications in aging and disease. Annu. Rev. Biochem. 80: 1089–1115. - PubMed
-
- Arrigo A. P., Simon S., Gibert B., Kretz-Remy C., Nivon M., et al. , 2007. Hsp27 (HspB1) and alphaB-crystallin (HspB5) as therapeutic targets. FEBS Lett. 581: 3665–3674. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

