Cell-free hemoglobin: a novel mediator of acute lung injury
- PMID: 26773065
- PMCID: PMC4796260
- DOI: 10.1152/ajplung.00155.2015
Cell-free hemoglobin: a novel mediator of acute lung injury
Abstract
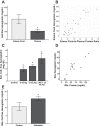
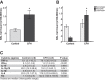
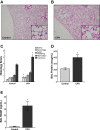
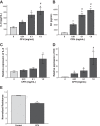
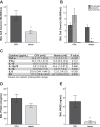
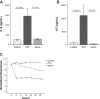
Patients with the acute respiratory distress syndrome (ARDS) have elevated levels of cell-free hemoglobin (CFH) in the air space, but the contribution of CFH to the pathogenesis of acute lung injury is unknown. In the present study, we demonstrate that levels of CFH in the air space correlate with measures of alveolar-capillary barrier dysfunction in humans with ARDS (r = 0.89, P < 0.001) and in mice with ventilator-induced acute lung injury (r = 0.89, P < 0.001). To investigate the specific contribution of CFH to ARDS, we studied the impact of purified CFH in the mouse lung and on cultured mouse lung epithelial (MLE-12) cells. Intratracheal delivery of CFH in mice causes acute lung injury with air space inflammation and alveolar-capillary barrier disruption. Similarly, in MLE-12 cells, CFH increases proinflammatory cytokine expression and increases paracellular permeability as measured by electrical cell-substrate impedance sensing. Next, to determine whether these effects are mediated by the iron-containing heme moiety of CFH, we treated mice with intratracheal hemin, the chloride salt of heme, and found that hemin was sufficient to increase alveolar permeability but failed to induce proinflammatory cytokine expression or epithelial cell injury. Together, these data identify CFH in the air space as a previously unrecognized driver of lung epithelial injury in human and experimental ARDS and suggest that CFH and hemin may contribute to ARDS through different mechanisms. Interventions targeting CFH and heme in the air space could provide a new therapeutic approach for ARDS.
Keywords: acute respiratory distress syndrome; air space inflammation; hemin; hemoglobin; lung epithelium; permeability.
Copyright © 2016 the American Physiological Society.
Figures
References
-
- Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet 2: 319–323, 1967. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL090785/HL/NHLBI NIH HHS/United States
- HL103836/HL/NHLBI NIH HHS/United States
- UL1 TR000445/TR/NCATS NIH HHS/United States
- R01 HL126671/HL/NHLBI NIH HHS/United States
- R21 HL117676/HL/NHLBI NIH HHS/United States
- UL1TR000445/TR/NCATS NIH HHS/United States
- R01 HL135849/HL/NHLBI NIH HHS/United States
- R01 HL126176/HL/NHLBI NIH HHS/United States
- K24 HL103836/HL/NHLBI NIH HHS/United States
- K08 HL090785/HL/NHLBI NIH HHS/United States
- HL087738/HL/NHLBI NIH HHS/United States
- RR024975/RR/NCRR NIH HHS/United States
- T32 HL087738/HL/NHLBI NIH HHS/United States
- UL1 RR024975/RR/NCRR NIH HHS/United States
- HL117676/HL/NHLBI NIH HHS/United States
- HL126671/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

