Inducible Expression of CXCL1 within the Central Nervous System Amplifies Viral-Induced Demyelination
- PMID: 26773148
- PMCID: PMC4742654
- DOI: 10.4049/jimmunol.1501802
Inducible Expression of CXCL1 within the Central Nervous System Amplifies Viral-Induced Demyelination
Abstract
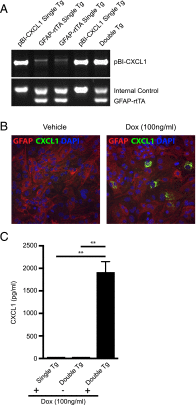
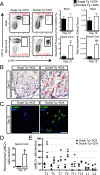
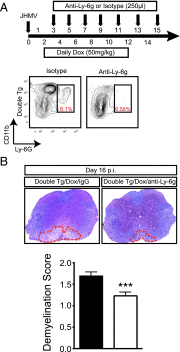
The functional role of the ELR(+) chemokine CXCL1 in host defense and disease following infection of the CNS with the neurotropic JHM strain of mouse hepatitis virus (JHMV) was examined. Mice in which expression of CXCL1 is under the control of a tetracycline-inducible promoter active within glial fibrillary acidic protein-positive cells were generated and this allowed for selectively increasing CNS expression of CXCL1 in response to JHMV infection and evaluating the effects on neuroinflammation, control of viral replication, and demyelination. Inducible expression of CNS-derived CXCL1 resulted in increased levels of CXCL1 protein within the serum, brain, and spinal cord that correlated with increased frequency of Ly6G(+)CD11b(+) neutrophils present within the CNS. Elevated levels of CXCL1 did not influence the generation of virus-specific T cells, and there was no difference in control of JHMV replication compared with control mice, indicating that T cell infiltration into the CNS is CXCL1-independent. Sustained CXCL1 expression within the CNS resulted in increased mortality that correlated with elevated neutrophil infiltration, diminished numbers of mature oligodendrocytes, and an increase in the severity of demyelination. Neutrophil ablation in CXCL1-transgenic mice reduced the severity of demyelination in mice, arguing for a role for these cells in white matter damage. Collectively, these findings illustrate that sustained CXCL1 expression amplifies the severity of white matter damage and that neutrophils can contribute to this process in a model of viral-induced neurologic disease.
Copyright © 2016 by The American Association of Immunologists, Inc.
Figures

Similar articles
-
Induced CNS expression of CXCL1 augments neurologic disease in a murine model of multiple sclerosis via enhanced neutrophil recruitment.Eur J Immunol. 2018 Jul;48(7):1199-1210. doi: 10.1002/eji.201747442. Epub 2018 May 16. Eur J Immunol. 2018. PMID: 29697856 Free PMC article.
-
Cystatin F attenuates neuroinflammation and demyelination following murine coronavirus infection of the central nervous system.J Neuroinflammation. 2024 Jun 15;21(1):157. doi: 10.1186/s12974-024-03153-0. J Neuroinflammation. 2024. PMID: 38879499 Free PMC article.
-
Sustained Infiltration of Neutrophils Into the CNS Results in Increased Demyelination in a Viral-Induced Model of Multiple Sclerosis.Front Immunol. 2022 Sep 29;13:931388. doi: 10.3389/fimmu.2022.931388. eCollection 2022. Front Immunol. 2022. PMID: 36248905 Free PMC article.
-
Chemokine CXCL10 and Coronavirus-Induced Neurologic Disease.Viral Immunol. 2019 Jan/Feb;32(1):25-37. doi: 10.1089/vim.2018.0073. Epub 2018 Aug 15. Viral Immunol. 2019. PMID: 30109979 Free PMC article. Review.
-
ELR(+) chemokine signaling in host defense and disease in a viral model of central nervous system disease.Front Cell Neurosci. 2014 Jun 17;8:165. doi: 10.3389/fncel.2014.00165. eCollection 2014. Front Cell Neurosci. 2014. PMID: 24987333 Free PMC article. Review.
Cited by
-
Daphnetin alleviates neuropathic pain in chronic constrictive injury rats via regulating the NF-κB dependent CXCL1/CXCR2 signaling pathway.Pharm Biol. 2023 Dec;61(1):746-754. doi: 10.1080/13880209.2023.2198560. Pharm Biol. 2023. PMID: 37177984 Free PMC article.
-
Neutrophils and viral-induced neurologic disease.Clin Immunol. 2018 Apr;189:52-56. doi: 10.1016/j.clim.2016.05.009. Epub 2016 Jun 8. Clin Immunol. 2018. PMID: 27288312 Free PMC article. Review.
-
CXCR2 Signaling and Remyelination in Preclinical Models of Demyelination.DNA Cell Biol. 2020 Jan;39(1):3-7. doi: 10.1089/dna.2019.5182. Epub 2019 Dec 17. DNA Cell Biol. 2020. PMID: 31851535 Free PMC article. Review.
-
T cell-selective deletion of Oct1 protects animals from autoimmune neuroinflammation while maintaining neurotropic pathogen response.J Neuroinflammation. 2019 Jul 3;16(1):133. doi: 10.1186/s12974-019-1523-3. J Neuroinflammation. 2019. PMID: 31266507 Free PMC article.
-
Innate Immune Responses and Viral-Induced Neurologic Disease.J Clin Med. 2018 Dec 20;8(1):3. doi: 10.3390/jcm8010003. J Clin Med. 2018. PMID: 30577473 Free PMC article. Review.
References
-
- Liu M. T., Chen B. P., Oertel P., Buchmeier M. J., Armstrong D., Hamilton T. A., Lane T. E. 2000. The T cell chemoattractant IFN-inducible protein 10 is essential in host defense against viral-induced neurologic disease. J. Immunol. 165: 2327–2330. - PubMed
-
- Lane T. E., Asensio V. C., Yu N., Paoletti A. D., Campbell I. L., Buchmeier M. J. 1998. Dynamic regulation of α- and β-chemokine expression in the central nervous system during mouse hepatitis virus-induced demyelinating disease. J. Immunol. 160: 970–978. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

