Feedback regulation of G protein-coupled receptor signaling by GRKs and arrestins
- PMID: 26773211
- PMCID: PMC4779377
- DOI: 10.1016/j.semcdb.2015.12.015
Feedback regulation of G protein-coupled receptor signaling by GRKs and arrestins
Abstract
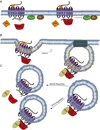
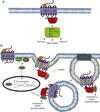
GPCRs are ubiquitous in mammalian cells and present intricate mechanisms for cellular signaling and communication. Mechanistically, GPCR signaling was identified to occur vectorially through heterotrimeric G proteins that are negatively regulated by GRK and arrestin effectors. Emerging evidence highlights additional roles for GRK and Arrestin partners, and establishes the existence of interconnected feedback pathways that collectively define GPCR signaling. GPCRs influence cellular dynamics and can mediate pathologic development, such as cancer and cardiovascular remolding. Hence, a better understanding of their overall signal regulation is of great translational interest and research continues to exploit the pharmacologic potential for modulating their activity.
Keywords: Arrestin; Biased signaling; G protein; GPCR; GRK; Signal transduction.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
G protein-coupled receptor interactions with arrestins and GPCR kinases: The unresolved issue of signal bias.J Biol Chem. 2022 Sep;298(9):102279. doi: 10.1016/j.jbc.2022.102279. Epub 2022 Jul 19. J Biol Chem. 2022. PMID: 35863432 Free PMC article. Review.
-
How Ligands Achieve Biased Signaling toward Arrestins.Biochemistry. 2025 Mar 4;64(5):967-977. doi: 10.1021/acs.biochem.4c00843. Epub 2025 Feb 12. Biochemistry. 2025. PMID: 39943784 Review.
-
Phosphorylation barcoding as a mechanism of directing GPCR signaling.Sci Signal. 2011 Aug 9;4(185):pe36. doi: 10.1126/scisignal.2002331. Sci Signal. 2011. PMID: 21868354 Review.
-
GRKs as Modulators of Neurotransmitter Receptors.Cells. 2020 Dec 31;10(1):52. doi: 10.3390/cells10010052. Cells. 2020. PMID: 33396400 Free PMC article. Review.
-
Chapter Three - Ubiquitination and Protein Turnover of G-Protein-Coupled Receptor Kinases in GPCR Signaling and Cellular Regulation.Prog Mol Biol Transl Sci. 2016;141:85-140. doi: 10.1016/bs.pmbts.2016.04.002. Epub 2016 May 7. Prog Mol Biol Transl Sci. 2016. PMID: 27378756 Review.
Cited by
-
Biased activation of β2-AR/Gi/GRK2 signal pathway attenuated β1-AR sustained activation induced by β1-adrenergic receptor autoantibody.Cell Death Discov. 2021 Nov 8;7(1):340. doi: 10.1038/s41420-021-00735-2. Cell Death Discov. 2021. PMID: 34750352 Free PMC article.
-
Inhibition of Human Prostate and Bladder Smooth Muscle Contraction, Vasoconstriction of Porcine Renal and Coronary Arteries, and Growth-Related Functions of Prostate Stromal Cells by Presumed Small Molecule Gαq/11 Inhibitor, YM-254890.Front Physiol. 2022 May 23;13:884057. doi: 10.3389/fphys.2022.884057. eCollection 2022. Front Physiol. 2022. PMID: 35677088 Free PMC article.
-
Editorial: G Protein-Coupled Receptor Kinases (GRKs) and β-Arrestins: New Insights Into Disease Regulators.Front Pharmacol. 2020 Jan 28;10:1654. doi: 10.3389/fphar.2019.01654. eCollection 2019. Front Pharmacol. 2020. PMID: 32047443 Free PMC article. No abstract available.
-
The LPA3 Receptor: Regulation and Activation of Signaling Pathways.Int J Mol Sci. 2021 Jun 23;22(13):6704. doi: 10.3390/ijms22136704. Int J Mol Sci. 2021. PMID: 34201414 Free PMC article. Review.
-
Dawn of a New RAMPage.Trends Pharmacol Sci. 2020 Apr;41(4):249-265. doi: 10.1016/j.tips.2020.01.009. Epub 2020 Feb 27. Trends Pharmacol Sci. 2020. PMID: 32115276 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

