A Bifunctional Electrocatalyst for Oxygen Evolution and Oxygen Reduction Reactions in Water
- PMID: 26773287
- PMCID: PMC4949709
- DOI: 10.1002/anie.201508404
A Bifunctional Electrocatalyst for Oxygen Evolution and Oxygen Reduction Reactions in Water
Abstract
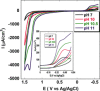
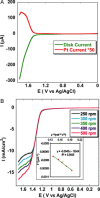
Oxygen reduction and water oxidation are two key processes in fuel cell applications. The oxidation of water to dioxygen is a 4 H(+)/4 e(-) process, while oxygen can be fully reduced to water by a 4 e(-)/4 H(+) process or partially reduced by fewer electrons to reactive oxygen species such as H2O2 and O2(-). We demonstrate that a novel manganese corrole complex behaves as a bifunctional catalyst for both the electrocatalytic generation of dioxygen as well as the reduction of dioxygen in aqueous media. Furthermore, our combined kinetic, spectroscopic, and electrochemical study of manganese corroles adsorbed on different electrode materials (down to a submolecular level) reveals mechanistic details of the oxygen evolution and reduction processes.
Keywords: corroles; electrochemistry; manganese; oxygen evolution; oxygen reduction.
© 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures





References
-
- None
-
- Galstyan A., Robertazzi A., Knapp E. W., J. Am. Chem. Soc. 2012, 134, 7442–7449; - PubMed
-
- Dasgupta J., van Willigen R. T., Dismukes G. C., Phys. Chem. Chem. Phys. 2004, 6, 4793–4802;
-
- Dismukes G. Charles, van Willigen R. T. in Encyclopedia of Inorganic Chemistry, Wiley, Hoboken, 2006;
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

