Pre-Existing Mature Oligodendrocytes Do Not Contribute to Remyelination following Toxin-Induced Spinal Cord Demyelination
- PMID: 26773350
- PMCID: PMC4816704
- DOI: 10.1016/j.ajpath.2015.11.005
Pre-Existing Mature Oligodendrocytes Do Not Contribute to Remyelination following Toxin-Induced Spinal Cord Demyelination
Abstract
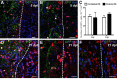
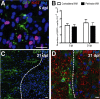
Remyelination is the regenerative response to demyelination. Although the oligodendrocyte progenitor is established as the major source of remyelinating cells, there is no conclusive evidence on whether mature, differentiated oligodendrocytes can also contribute to remyelination. Using two different inducible myelin-CreER mouse strains in which mature oligodendrocytes were prelabeled by the expression of membrane-bound Green fluorescent protein, we found that after focal spinal cord demyelination, the surrounding surviving labeled oligodendrocytes did not proliferate but remained at a consistent density. Furthermore, existing (prelabeled) oligodendrocytes showed no evidence of incorporation or migration into the lesioned area, or of process extension from the peripheral margins into the lesion. Thus, mature oligodendrocytes do not normally contribute to remyelination and are therefore not a promising target for regenerative therapy.
Copyright © 2016 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Zawadzka M., Rivers L.E., Fancy S.P., Zhao C., Tripathi R., Jamen F., Young K., Goncharevich A., Pohl H., Rizzi M., Rowitch D.H., Kessaris N., Suter U., Richardson W.D., Franklin R.J.M. CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell. 2010;6:578–590. - PMC - PubMed
-
- Ludwin S.K. Proliferation of mature oligodendrocytes after trauma to the central nervous system. Nature. 1984;308:274–275. - PubMed
-
- Wood P.M., Bunge R.P. Evidence that axons are mitogenic for oligodendrocytes isolated from adult animals. Nature. 1986;320:756–758. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

