Roles of phosphodiesterases in the regulation of the cardiac cyclic nucleotide cross-talk signaling network
- PMID: 26773602
- PMCID: PMC4764497
- DOI: 10.1016/j.yjmcc.2016.01.004
Roles of phosphodiesterases in the regulation of the cardiac cyclic nucleotide cross-talk signaling network
Abstract
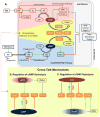
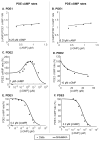
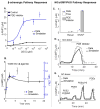
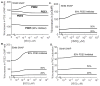
The balanced signaling between the two cyclic nucleotides (cNs) cAMP and cGMP plays a critical role in regulating cardiac contractility. Their degradation is controlled by distinctly regulated phosphodiesterase isoenzymes (PDEs), which in turn are also regulated by these cNs. As a result, PDEs facilitate communication between the β-adrenergic and Nitric Oxide (NO)/cGMP/Protein Kinase G (PKG) signaling pathways, which regulate the synthesis of cAMP and cGMP respectively. The phenomena in which the cAMP and cGMP pathways influence the dynamics of each other are collectively referred to as cN cross-talk. However, the cross-talk response and the individual roles of each PDE isoenzyme in shaping this response remain to be fully characterized. We have developed a computational model of the cN cross-talk network that mechanistically integrates the β-adrenergic and NO/cGMP/PKG pathways via regulation of PDEs by both cNs. The individual model components and the integrated network model replicate experimentally observed activation-response relationships and temporal dynamics. The model predicts that, due to compensatory interactions between PDEs, NO stimulation in the presence of sub-maximal β-adrenergic stimulation results in an increase in cytosolic cAMP accumulation and corresponding increases in PKA-I and PKA-II activation; however, the potentiation is small in magnitude compared to that of NO activation of the NO/cGMP/PKG pathway. In a reciprocal manner, β-adrenergic stimulation in the presence of sub-maximal NO stimulation results in modest cGMP elevation and corresponding increase in PKG activation. In addition, we demonstrate that PDE2 hydrolyzes increasing amounts of cAMP with increasing levels of β-adrenergic stimulation, and hydrolyzes increasing amounts of cGMP with decreasing levels of NO stimulation. Finally, we show that PDE2 compensates for inhibition of PDE5 both in terms of cGMP and cAMP dynamics, leading to cGMP elevation and increased PKG activation, while maintaining whole-cell β-adrenergic responses similar to that prior to PDE5 inhibition. By defining and quantifying reactions comprising cN cross-talk, the model characterizes the cross-talk response and reveals the underlying mechanisms of PDEs in this non-linear, tightly-coupled reaction system.
Keywords: Cardiac myocytes; Computational model; Cyclic nucleotide cross-talk signaling network; NO/cGMP/PKG pathway; Phosphodiesterases; β-adrenergic pathway.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures




References
-
- Fischmeister R, Castro LR, Abi-Gerges A, Rochais F, Jurevičius J, Leroy J, et al. Compartmentation of Cyclic Nucleotide Signaling in the Heart The Role of Cyclic Nucleotide Phosphodiesterases. Circulation Research. 2006;99:816–28. - PubMed
-
- Mika D, Leroy J, Vandecasteele G, Fischmeister R. PDEs create local domains of cAMP signaling. Journal of molecular and cellular cardiology. 2012;52:323–9. - PubMed
-
- Zaccolo M, Movsesian MA. cAMP and cGMP Signaling Cross-Talk Role of Phosphodiesterases and Implications for Cardiac Pathophysiology. Circulation Research. 2007;100:1569–78. - PubMed
-
- Stangherlin A, Zaccolo M. cGMP-cAMP interplay in cardiac myocytes: a local affair with far-reaching consequences for heart function. Biochem Soc Trans. 2012;40:11–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

