The effect of comorbidity on the use of adjuvant chemotherapy and type of regimen for curatively resected stage III colon cancer patients
- PMID: 26773804
- PMCID: PMC4864816
- DOI: 10.1002/cam4.632
The effect of comorbidity on the use of adjuvant chemotherapy and type of regimen for curatively resected stage III colon cancer patients
Abstract
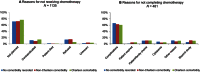
Postsurgical chemotherapy is guideline-recommended therapy for stage III colon cancer patients. Factors associated with patients not receiving adjuvant chemotherapy were identified in numerous studies; comorbidity was recognized as an important factor besides patient's age. We assessed the association between comorbidity and the use of adjuvant chemotherapy and type of chemotherapy regimen. Stage III colon cancer patients who underwent surgical resection were obtained from ten Centers for Disease Control and Prevention (CDC)-NPCR Specialized Registries which participated in the Comparative Effectiveness Research (CER) project. Comorbidity was classified into no comorbidity recorded, Charlson, non-Charlson comorbidities, number, and severity of Charlson comorbidity. Pearson chi-square test and multivariable logistic regression were employed. Of 3180 resected stage III colon cancer patients, 64% received adjuvant chemotherapy. After adjusting for patient's demographic and tumor characteristics, there were no significant differences in receipt of chemotherapy between Charlson and non-Charlson comorbidity. However, patients who had two or more Charlson comorbidities or had moderate to severe disease were significantly less likely to have chemotherapy (ORs 0.69 [95% CI, 0.51-0.92] and 0.62 [95% CI, 0.42-0.91], respectively) when compared with those with non-Charlson comorbidity. In addition, those with moderate or severe comorbidities were more likely to receive single chemotherapy agent (P < 0.0001). Capecitabine and FOLFOX were the most common single- and multi-agent regimens regardless of type of comorbidity grouping. Both the number and severity of comorbidity were significantly associated with receipt of guideline-recommended chemotherapy and type of agent in stage III resected colon cancer patients. Better personalized care based on individual patient's condition ought to be recognized.
Keywords: Adjuvant chemotherapy; chemotherapy regimen; colon cancer; comorbidity; treatment.
© 2016 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Figures

Similar articles
-
Practical outcome of adjuvant FOLFOX4 chemotherapy in elderly patients with stage III colon cancer: single-center study in Korea.Jpn J Clin Oncol. 2013 Feb;43(2):132-8. doi: 10.1093/jjco/hys195. Epub 2012 Nov 29. Jpn J Clin Oncol. 2013. PMID: 23204187
-
Utilization and impact of adjuvant chemotherapy among patients with resected stage II colon cancer: a multi-institutional analysis.J Surg Res. 2017 Jul;215:12-20. doi: 10.1016/j.jss.2017.03.017. Epub 2017 Mar 31. J Surg Res. 2017. PMID: 28688636 Free PMC article.
-
Effect of Comorbidities in Stage II/III Colorectal Cancer Patients Treated With Surgery and Neoadjuvant/Adjuvant Chemotherapy: A Single-Center, Observational Study.Clin Colorectal Cancer. 2018 Sep;17(3):e489-e498. doi: 10.1016/j.clcc.2018.03.010. Epub 2018 Mar 21. Clin Colorectal Cancer. 2018. PMID: 29650416
-
[Adjuvant chemotherapy for colon cancer].Tidsskr Nor Laegeforen. 2007 Nov 29;127(23):3094-6. Tidsskr Nor Laegeforen. 2007. PMID: 18049502 Review. Norwegian.
-
Adjuvant Chemotherapy for Stage II Colon Cancer: A Clinical Dilemma.J Oncol Pract. 2017 Apr;13(4):233-241. doi: 10.1200/JOP.2016.017210. J Oncol Pract. 2017. PMID: 28399381 Review.
Cited by
-
The Association between Chronic Conditions, End-of-Life Health Care Use, and Documentation of Advance Care Planning among Patients with Cancer.J Palliat Med. 2020 Oct;23(10):1335-1341. doi: 10.1089/jpm.2019.0530. Epub 2020 Mar 16. J Palliat Med. 2020. PMID: 32181689 Free PMC article.
-
The prognostic and predictive significance of perineural invasion in stage I to III colon cancer: a propensity score matching-based analysis.World J Surg Oncol. 2024 May 11;22(1):129. doi: 10.1186/s12957-024-03405-6. World J Surg Oncol. 2024. PMID: 38734718 Free PMC article.
-
Age-adjusted charlson comorbidity index and 30-day morbidity in pelvic surgeries.South Asian J Cancer. 2018 Oct-Dec;7(4):240-243. doi: 10.4103/sajc.sajc_241_17. South Asian J Cancer. 2018. PMID: 30430092 Free PMC article.
-
Factors associated with the survival of colorectal cancer in Mexico.Intest Res. 2020 Jul;18(3):315-324. doi: 10.5217/ir.2019.09179. Epub 2020 May 19. Intest Res. 2020. PMID: 32418415 Free PMC article.
-
Electrochemical and optical biosensors for early-stage cancer diagnosis by using graphene and graphene oxide.Cancer Nanotechnol. 2017;8(1):10. doi: 10.1186/s12645-017-0035-z. Epub 2017 Dec 11. Cancer Nanotechnol. 2017. PMID: 29250208 Free PMC article. Review.
References
-
- Moertel, C. G. , Fleming T. R., Macdonald J. S., et al. 1995. Fluorouracil plus levamisole as effective adjuvant therapy after resection of stage III colon carcinoma: a final report. Ann. Intern. Med. 122:321–326. - PubMed
-
- O'Connell, M. J. , Mailliard J. A., Kahn M. J., et al. 1997. Controlled trial of fluorouracil and low‐dose leucovorin given for 6 months as postoperative adjuvant therapy for colon cancer. J. Clin. Oncol. 15:246–250. - PubMed
-
- Gill, S. , Loprinzi C. L., Sargent D. J., et al. 2004. Pooled analysis of fluorouracil‐based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J. Clin. Oncol. 22:1806–1979. - PubMed
-
- Andre, T. , Boni C., Mounedji‐Boudiaf L., et al. 2004. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N. Engl. J. Med. 350:2343–2351. - PubMed
-
- Sargent, D. J. , Goldberg R. M., Jacobson S. D., et al. 2001. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N. Engl. J. Med. 345:1091–1097. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

