Diffusion MRI in early cancer therapeutic response assessment
- PMID: 26773848
- PMCID: PMC4947029
- DOI: 10.1002/nbm.3458
Diffusion MRI in early cancer therapeutic response assessment
Abstract
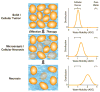
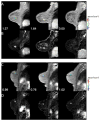
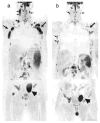
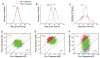
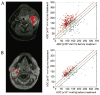
Imaging biomarkers for the predictive assessment of treatment response in patients with cancer earlier than standard tumor volumetric metrics would provide new opportunities to individualize therapy. Diffusion-weighted MRI (DW-MRI), highly sensitive to microenvironmental alterations at the cellular level, has been evaluated extensively as a technique for the generation of quantitative and early imaging biomarkers of therapeutic response and clinical outcome. First demonstrated in a rodent tumor model, subsequent studies have shown that DW-MRI can be applied to many different solid tumors for the detection of changes in cellularity as measured indirectly by an increase in the apparent diffusion coefficient (ADC) of water molecules within the lesion. The introduction of quantitative DW-MRI into the treatment management of patients with cancer may aid physicians to individualize therapy, thereby minimizing unnecessary systemic toxicity associated with ineffective therapies, saving valuable time, reducing patient care costs and ultimately improving clinical outcome. This review covers the theoretical basis behind the application of DW-MRI to monitor therapeutic response in cancer, the analytical techniques used and the results obtained from various clinical studies that have demonstrated the efficacy of DW-MRI for the prediction of cancer treatment response. Copyright © 2016 John Wiley & Sons, Ltd.
Keywords: cancer treatment response; diffusion-weighted MRI; functional diffusion map; imaging biomarker; review article.
Copyright © 2016 John Wiley & Sons, Ltd.
Figures
References
-
- Gehan EA, et al. Historical and methodological developments in clinical trials at the National Cancer Institute. Stat Med. 1990;9(8):871–80. discussion 903–906. - PubMed
-
- Eisenhauer EA, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–47. - PubMed
-
- Wen PY, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–72. - PubMed
-
- World Health Organization (WHO) WHO Handbook for Reporting Results of Cancer Treatment. Geneva: WHO; 1979.
-
- Jaffe CC. Measures of response: RECIST, WHO, and new alternatives. J Clin Oncol. 2006;24(20):3245–51. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

