A Comparative Pharmacokinetic Study of Myrislignan by UHPLC-MS After Oral Administration of a Monomer and Myristica fragrans Extract to Rats
- PMID: 26774114
- PMCID: PMC4890437
- DOI: 10.1093/chromsci/bmv227
A Comparative Pharmacokinetic Study of Myrislignan by UHPLC-MS After Oral Administration of a Monomer and Myristica fragrans Extract to Rats
Abstract
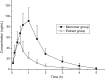
An ultra-high performance liquid chromatography coupled with mass spectrometry (UHPLC-MS) method was developed and validated to quantify myrislignan in rat plasma using podophyllotoxin as an internal standard (IS). The chromatographic separation of myrislignan and IS was performed on a 3.0 µm Hypersil C18 column (50 mm × 4.6 mm) with methanol and water containing 0.1% acetic acid (80:20, v/v) as the mobile phase at a flow rate of 0.3 mL/min. An electrospray ionization was used in the positive selective-ion monitoring mode for the target ions at m/z 397 and m/z 437 for the quantification of myrislignan and IS. The total run time was 3.6 min for each run. The calibration curve was linear over the range of 0.75-300 ng/mL (r> 0.995) with the lower limit of quantitation at 0.75 ng/mL. Intra- and interday precision was below 11.49%, and the mean accuracy ranged from -9.75 to 7.45%. The proposed method was successfully applied to evaluate the pharmacokinetic properties of myrislignan after oral administration of the myrislignan monomer and Myristica fragrans extract in rats. Statistical analyses indicate that the pharmacokinetic properties of myrislignan in rats have significant differences between two groups.
© The Author 2016. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures



Similar articles
-
Determination and pharmacokinetic study of two triterpenoid saponins in rat plasma after oral administration of the extract of Aralia elata leaves by UHPLC-ESI-MS/MS.J Chromatogr B Analyt Technol Biomed Life Sci. 2015 Mar 15;985:164-71. doi: 10.1016/j.jchromb.2015.01.036. Epub 2015 Feb 3. J Chromatogr B Analyt Technol Biomed Life Sci. 2015. PMID: 25687802
-
Determination of geniposide in adjuvant arthritis rat plasma by ultra-high performance liquid chromatography tandem mass spectrometry method and its application to oral bioavailability and plasma protein binding ability studies.J Pharm Biomed Anal. 2015 Apr 10;108:122-8. doi: 10.1016/j.jpba.2015.01.044. Epub 2015 Feb 7. J Pharm Biomed Anal. 2015. PMID: 25771205
-
Determination and pharmacokinetic study of four xanthones in rat plasma after oral administration of Gentianella acuta extract by UHPLC-ESI-MS/MS.J Ethnopharmacol. 2015 Nov 4;174:261-9. doi: 10.1016/j.jep.2015.08.023. Epub 2015 Aug 20. J Ethnopharmacol. 2015. PMID: 26297839
-
Ultra high performance liquid chromatography tandem mass spectrometry method for the simultaneous determination of multiple bioactive constituents in fruit extracts of Myristica fragrans and its marketed polyherbal formulations using a polarity switching technique.J Sep Sci. 2015 May;38(8):1277-85. doi: 10.1002/jssc.201401297. Epub 2015 Mar 25. J Sep Sci. 2015. PMID: 25631526
-
Rapid determination of canagliflozin in rat plasma by UHPLC-MS/MS using negative ionization mode to avoid adduct-ions formation.Talanta. 2015 Jan;132:29-36. doi: 10.1016/j.talanta.2014.08.041. Epub 2014 Aug 23. Talanta. 2015. PMID: 25476275
Cited by
-
Determination of myrislignan levels in BALB/c mouse plasma by LC-MS/MS and a comparison of its pharmacokinetics after oral and intraperitoneal administration.BMC Vet Res. 2021 Aug 16;17(1):275. doi: 10.1186/s12917-021-02990-y. BMC Vet Res. 2021. PMID: 34399756 Free PMC article.
-
Study of Absorption Characteristics of the Total Saponins from Radix Ilicis Pubescentis in an In Situ Single-Pass Intestinal Perfusion (SPIP) Rat Model by Using Ultra Performance Liquid Chromatography (UPLC).Molecules. 2017 Nov 1;22(11):1867. doi: 10.3390/molecules22111867. Molecules. 2017. PMID: 29104273 Free PMC article.
References
-
- The State Pharmacopoeia Commission of P.R. China. Pharmacopoeia of the P.R. China. Chemical Industry Press, Beijing, China, (2010), pp. 126–127.
-
- Olajide O.A., Ajayi F.F., Ekhelar A.I., Awe S.O., Makinde J.M., Alada A.R.; Biological effects of Myristica fragrans (Nutmeg) extract; Phytotherapy Research, (1999); 13: 344–345. - PubMed
-
- Chatterjee S., Niaz Z., Gautam S., Adhikari S., Variyar P.S., Sharma A.; Antioxidant activity of some phenolic constituents from green pepper (Piper nigrum L.) and fresh nutmeg mace (Myristica fragrans); Food Chemistry, (2007); 101: 515–523.
-
- Piaru S.P., Mahmud R., Abdul Majid A.M., Mahmoud Nassar Z.D.; Antioxidant and antiangiogenic activities of the essential oils of Myristica fragrans and Morinda citrifolia; Asian Pacific Journal of Tropical Medicine, (2012); 5: 294–298. - PubMed
-
- Cuong T.D., Hung T.M., Na M., Ha do T., Kim J.C., Lee D. et al. ; Inhibitory effect on NO production of phenolic compounds from Myristica fragrans; Bioorganic & Medicinal Chemistry Letters, (2011); 21: 6884–6887. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

