1,25-Dihydroxyvitamin D induces the glutamate transporter SLC1A1 and alters glutamate handling in non-transformed mammary cells
- PMID: 26774511
- PMCID: PMC4779372
- DOI: 10.1016/j.mce.2016.01.011
1,25-Dihydroxyvitamin D induces the glutamate transporter SLC1A1 and alters glutamate handling in non-transformed mammary cells
Abstract
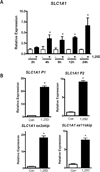
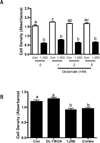
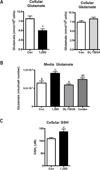
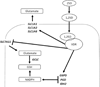
Genomic profiling of immortalized human mammary epithelial (hTERT-HME1) cells identified several metabolic genes, including the membrane glutamate transporter, SLC1A1, as 1,25-dihydroxyvitamin D3 (1,25D) regulated. In these studies we have surveyed the effects of 1,25D on known glutamate transporters and evaluated its impact on cellular glutamate handling. We confirm that expression of SLC1A1 and all of its known transcript variants are significantly upregulated in hTERT-HME1 cells following 1,25D treatment. Expression of the full-length cognate protein, EAAT3, is correspondingly increased in 1,25D treated hTERT-HME1 cells. Under the same conditions, the expression of two other glutamate transporters--SLC1A6 (EAAT4) and SLC1A2 (EAAT2 or GLT-1)--is enhanced by 1,25D while that of SLC1A3 (EAAT1 or GLAST) and SLC7A11 (xCT) is decreased. Glutamate is not essential for growth of hTERT-HME1 cells, and supplemental glutamate (up to 0.5 mM) does not abrogate the growth inhibitory effects of 1,25D. These data suggest that extracellular glutamate is not a major contributor to cellular energy metabolism in hTERT-HME1 cells under basal conditions and that the growth inhibitory effects of 1,25D are not secondary to its effects on glutamate handling. Instead, the effects of 1,25D on glutamate transporters translated to a decrease in cellular glutamate concentration and an increase in media glutamate concentration, suggesting that one or more of these transporters functions to export glutamate in response to 1,25D exposure. The reduced cellular glutamate concentration may also reflect its incorporation into the cellular glutathione (GSH) pool, which is increased upon 1,25D treatment. In support of this concept, the expression of GCLC (which codes for the rate-limiting enzyme in GSH synthesis) and genes which generate reducing equivalents in the form of NADPH (ie, G6PD, PGD, IDH2) are elevated in 1,25D-treated cells. Taken together, these data identify 1,25D as a physiological regulator of multiple membrane glutamate transporters that impacts on overall cellular glutamate handling.
Keywords: Glutamate; Glutamine; Glutathione; Mammary cells; Vitamin D.
Copyright © 2016 Elsevier Ireland Ltd. All rights reserved.
Figures




References
-
- Chakrabarty K, Bhattacharyya S, Christopher R, Khanna S. Glutamatergic dysfunction in OCD. Neuropsychopharmacology. 2005 Sep;30(9):1735–1740. - PubMed
-
- Hynd MR, Scott HL, Dodd PR. Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer's disease. Neurochem Int. 2004 Oct;45(5):583–595. - PubMed
-
- Budczies J, Pfitzner BM, Gyorffy B, Winzer KJ, Radke C, Dietel M, et al. Glutamate enrichment as new diagnostic opportunity in breast cancer. Int J Cancer. 2015 Apr 1;136(7):1619–1628. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

