Lipidomics and H2(18)O labeling techniques reveal increased remodeling of DHA-containing membrane phospholipids associated with abnormal locomotor responses in α-tocopherol deficient zebrafish (danio rerio) embryos
- PMID: 26774753
- PMCID: PMC4732018
- DOI: 10.1016/j.redox.2016.01.004
Lipidomics and H2(18)O labeling techniques reveal increased remodeling of DHA-containing membrane phospholipids associated with abnormal locomotor responses in α-tocopherol deficient zebrafish (danio rerio) embryos
Abstract
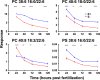
We hypothesized that vitamin E (α-tocopherol) is required by the developing embryonic brain to prevent depletion of highly polyunsaturated fatty acids, especially docosahexaenoic acid (DHA, 22:6), the loss of which we predicted would underlie abnormal morphological and behavioral outcomes. Therefore, we fed adult 5D zebrafish (Danio rerio) defined diets without (E-) or with added α-tocopherol (E+, 500mg RRR-α-tocopheryl acetate/kg diet) for a minimum of 80 days, and then spawned them to obtain E- and E+ embryos. The E- compared with E+ embryos were 82% less responsive (p<0.01) to a light/dark stimulus at 96h post-fertilization (hpf), demonstrating impaired locomotor behavior, even in the absence of gross morphological defects. Evaluation of phospholipid (PL) and lysophospholipid (lyso-PL) composition using untargeted lipidomics in E- compared with E+ embryos at 24, 48, 72, and 120hpf showed that four PLs and three lyso-PLs containing docosahexaenoic acid (DHA), including lysophosphatidylcholine (LPC 22:6, required for transport of DHA into the brain, p<0.001), were at lower concentrations in E- at all time-points. Additionally, H2(18)O labeling experiments revealed enhanced turnover of LPC 22:6 (p<0.001) and three other DHA-containing PLs in the E- compared with the E+ embryos, suggesting that increased membrane remodeling is a result of PL depletion. Together, these data indicate that α-tocopherol deficiency in the zebrafish embryo causes the specific depletion and increased turnover of DHA-containing PL and lyso-PLs, which may compromise DHA delivery to the brain and thereby contribute to the functional impairments observed in E- embryos.
Keywords: Brain; Development; Docosahexaenoic acid; H(2)(18)O; Mass spectrometry; Peroxidation; Phospholipids; Vitamin E.
Copyright © 2016 The Authors. Published by Elsevier B.V. All rights reserved.
Figures







References
-
- Westerfield M. University of Oregon Press; Eugene: 2007. The Zebrafish Book; A Guide for the Laboratory use of Zebrafish (Danio Rerio)
-
- Burton G.W., Joyce A., Ingold K.U. First proof that vitamin E is major lipid-soluble, chain-breaking antioxidant in human blood plasma. Lancet. 1982;2:327. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

