Is adjunctive pharmacotherapy in attention-deficit/hyperactivity disorder cost-effective in Canada: a cost-effectiveness assessment of guanfacine extended-release as an adjunctive therapy to a long-acting stimulant for the treatment of ADHD
- PMID: 26774811
- PMCID: PMC4715876
- DOI: 10.1186/s12888-016-0708-x
Is adjunctive pharmacotherapy in attention-deficit/hyperactivity disorder cost-effective in Canada: a cost-effectiveness assessment of guanfacine extended-release as an adjunctive therapy to a long-acting stimulant for the treatment of ADHD
Abstract
Background: Attention-deficit/hyperactivity disorder (ADHD) is a common psychiatric disorder in children, with worldwide prevalence of ADHD varying from 5.9 to 7.1 %, depending on the reporter. In case of inadequate response to stimulants, combination therapy of stimulants and an adjunctive medication may improve the control of ADHD symptoms, reduce the dose-limiting adverse events, and help control comorbidities. To date, the only medication to be used for adjunctive therapy to psychostimulants is guanfacine extended release (GXR). The aim of this study was to assess the economic impact of GXR as an adjunct therapy with long-acting stimulants (GXR + stimulant) compared to long-acting stimulant monotherapy (stimulant alone) in the treatment of children and adolescents with ADHD in Canada.
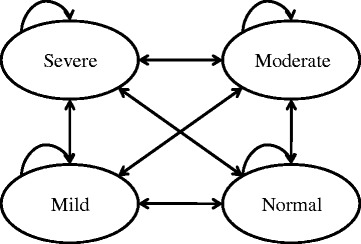
Method: A Markov model was developed using health states defined based on the clinician-reported Clinical Global Impression-Severity (CGI-S) score (normal, mild, moderate, severe). Transition probabilities were calculated based on patient-level data from a published study. Long-acting stimulants available in Canada were considered in the base-case model: amphetamine mixed salts, methylphenidate HCl formulations, and lisdexamfetamine dimesylate. Analyses were conducted from a Canadian Ministry of Health (MoH; Ontario) and a societal perspective over a 1-year time horizon with weekly cycles.
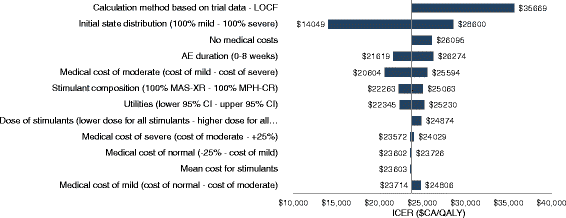
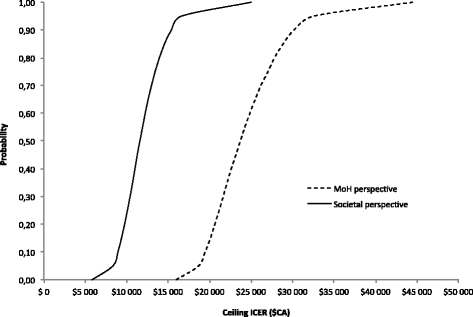
Results: Over a 1-year time horizon, GXR + stimulant was associated with 0.655 quality-adjusted life year (QALY), compared to 0.627 QALY with stimulant alone, for a gain of 0.028 QALY. From a MoH perspective, GXR+ stimulant and stimulant alone were associated with total costs of $CA1,617 and $CA949, respectively (difference of $CA668), which resulted in an incremental cost-effectiveness ratio (ICER) of $CA23,720/QALY. From a societal perspective, GXR + stimulant and stimulant alone were associated with total costs of $CA3,915 and $CA3,582, respectively (difference of $CA334), which resulted in an ICER of $CA11,845/QALY. Probabilistic sensitivity analysis (PSA) of GXR + stimulant showed that it remains a cost-effective strategy in 100 % of the simulations from both perspectives in numerous PSA and one-way sensitivity analyses, relative to a willingness to pay threshold of $50,000/QALY.
Conclusions: This economic evaluation demonstrates that GXR + stimulant is cost-effective compared to stimulant alone in the treatment of children and adolescents with ADHD in Canada.
Figures
Similar articles
-
Cost effectiveness of guanfacine extended release as an adjunctive therapy to a stimulant compared with stimulant monotherapy for the treatment of attention-deficit hyperactivity disorder in children and adolescents.Pharmacoeconomics. 2012 Aug 1;30(8):e1-15. doi: 10.2165/11632920-000000000-00000. Pharmacoeconomics. 2012. PMID: 22788263 Free PMC article. Clinical Trial.
-
Cost effectiveness of guanfacine extended-release versus atomoxetine for the treatment of attention-deficit/hyperactivity disorder: application of a matching-adjusted indirect comparison.Appl Health Econ Health Policy. 2012 Nov 1;10(6):381-95. doi: 10.1007/BF03261873. Appl Health Econ Health Policy. 2012. PMID: 23113551 Clinical Trial.
-
Guanfacine extended release as adjunctive therapy to psychostimulants in children and adolescents with attention-deficit/hyperactivity disorder.Adv Ther. 2012 May;29(5):385-400. doi: 10.1007/s12325-012-0020-1. Epub 2012 May 18. Adv Ther. 2012. PMID: 22610723 Review.
-
Safety and effectiveness of coadministration of guanfacine extended release and psychostimulants in children and adolescents with attention-deficit/hyperactivity disorder.J Child Adolesc Psychopharmacol. 2009 Oct;19(5):501-10. doi: 10.1089/cap.2008.0152. J Child Adolesc Psychopharmacol. 2009. PMID: 19877974 Free PMC article. Clinical Trial.
-
Evaluation of the current data on guanfacine extended release for the treatment of ADHD in children and adolescents.Expert Opin Pharmacother. 2020 Mar;21(4):417-426. doi: 10.1080/14656566.2019.1706480. Epub 2020 Jan 23. Expert Opin Pharmacother. 2020. PMID: 31971448 Review.
Cited by
-
Descriptive Cost-Effectiveness Analysis of a Counseling and Coordination Model in Psychosocial Care. Integration of Health Care and Social Rehabilitation.Front Psychiatry. 2020 Feb 12;10:1008. doi: 10.3389/fpsyt.2019.01008. eCollection 2019. Front Psychiatry. 2020. PMID: 32116823 Free PMC article.
-
Treatment Patterns, Health Care Resource Utilization, and Health Care Cost Associated with Atypical Antipsychotics or Guanfacine Extended Release in Children and Adolescents with Attention-Deficit/Hyperactivity Disorder in Quebec, Canada.J Child Adolesc Psychopharmacol. 2019 Dec;29(10):730-739. doi: 10.1089/cap.2019.0097. Epub 2019 Sep 17. J Child Adolesc Psychopharmacol. 2019. PMID: 31433205 Free PMC article.
References
-
- Brault MC, Lacourse E. Prevalence of prescribed attention-deficit hyperactivity disorder medications and diagnosis among Canadian preschoolers and school-age children: 1994–2007. Can J Psychiatry. 2012;57(2):93–101. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

