Synthesis and evaluation of vitamin D receptor-mediated activities of cholesterol and vitamin D metabolites
- PMID: 26774929
- PMCID: PMC4737989
- DOI: 10.1016/j.ejmech.2016.01.002
Synthesis and evaluation of vitamin D receptor-mediated activities of cholesterol and vitamin D metabolites
Abstract
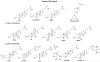
A systematic study with phase 1 and phase 2 metabolites of cholesterol and vitamin D was conducted to determine whether their biological activity is mediated by the vitamin D receptor (VDR). The investigation necessitated the development of novel synthetic routes for lithocholic acid (LCA) glucuronides (Gluc). Biochemical and cell-based assays were used to demonstrate that hydroxylated LCA analogs were not able to bind VDR. This excludes VDR from mediating their biological and pharmacological activities. Among the synthesized LCA conjugates a novel VDR agonist was identified. LCA Gluc II increased the expression of CYP24A1 in DU145 cancer cells especially in the presence of the endogenous VDR ligand 1,25(OH)2D3. Furthermore, the methyl ester of LCA was identified as novel VDR antagonist. For the first time, we showed that calcitroic acid, the assumed inactive final metabolite of vitamin D, was able to activate VDR-mediated transcription to a higher magnitude than bile acid LCA. Due to a higher metabolic stability in comparison to vitamin D, a very low toxicity, and high concentration in bile and intestine, calcitroic acid is likely to be an important mediator of the protective vitamin D properties against colon cancer.
Keywords: 1,25(OH)(2)D(3); CYP24A1; Calcitroic acid; DU145; Lithocholic acid; Metabolites; Vitamin D receptor.
Copyright © 2016 Elsevier Masson SAS. All rights reserved.
Figures
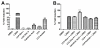
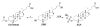

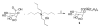
References
-
- Glass CK, Rosenfeld MG. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 2000;14:121–141. - PubMed
-
- Makishima M, Lu TT, Xie W, Whitfield GK, Domoto H, Evans RM. Vitamin D receptor as an intestinal bile acid sensor. Science. 2002;296:1313–1316. - PubMed
-
- Russell DW. The enzymes, regulation and genetics of bile acid synthesis. Annual Review of Biochemistry. 2003;72:137–174. - PubMed
-
- Ridlon JM, Kang D-J, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 2006;47:241–259. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

