Ginkgolide B protects human umbilical vein endothelial cells against xenobiotic injuries via PXR activation
- PMID: 26775663
- PMCID: PMC4753368
- DOI: 10.1038/aps.2015.124
Ginkgolide B protects human umbilical vein endothelial cells against xenobiotic injuries via PXR activation
Abstract
Aim: Pregnane X receptor (PXR) is a nuclear receptor that regulates a number of genes encoding drug metabolism enzymes and transporters and plays a key role in xeno- and endobiotic detoxification. Ginkgolide B has shown to increase the activity of PXR. Here we examined whether ginkgolide B activated PXR and attenuated xenobiotic-induced injuries in endothelial cells.
Methods: Human umbilical vein endothelial cells (HUVECs) were treated with ginkgolide B. The expression of PXR, CYP3A4, MDR1, VCAM-1, E-selectin and caspase-3 were quantified with qRT-PCR and Western blot analysis. Cell apoptosis was analyzed with flow cytometry. Fluorescently labeled human acute monocytic leukemia cells (THP-1 cells) were used to examine cell adhesion.
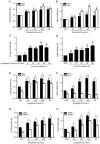
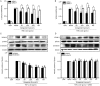
Results: Ginkgolide B (30-300 μmol/L) did not change the mRNA and protein levels of PXR in the cells, but dose-dependently increased nuclear translocation of PXR protein. Ginkgolide B increased the expression of CYP3A4 and MDR1 in the cells, which was partially reversed by pretreatment with the selective PXR signaling antagonist sulforaphane, or transfection with PXR siRNA. Functionally, ginkgolide B dose-dependently attenuated doxorubicin- or staurosporine-induced apoptosis, which was reversed by transfection with PXR siRNA. Moreover, ginkgolide B suppressed TNF-α-induced THP-1 cell adhesion and TNF-α-induced expression of vascular adhesion molecule 1 (VCAM-1) and E-selectin in the cells, which was also reversed by transfection with PXR siRNA.
Conclusion: Ginkgolide B exerts anti-apoptotic and anti-inflammatory effects on endothelial cells via PXR activation, suggesting that a PXR-mediated endothelial detoxification program may be important for protecting endothelial cells from xeno- and endobiotic-induced injuries.
Figures



Similar articles
-
Human pregnane X receptor agonism by Ginkgo biloba extract: assessment of the role of individual ginkgolides.J Pharmacol Exp Ther. 2010 Dec;335(3):771-80. doi: 10.1124/jpet.110.172338. Epub 2010 Aug 25. J Pharmacol Exp Ther. 2010. PMID: 20739453
-
Shear stress activation of nuclear receptor PXR in endothelial detoxification.Proc Natl Acad Sci U S A. 2013 Aug 6;110(32):13174-9. doi: 10.1073/pnas.1312065110. Epub 2013 Jul 22. Proc Natl Acad Sci U S A. 2013. PMID: 23878263 Free PMC article.
-
Ginkgolide B Suppresses TLR4-Mediated Inflammatory Response by Inhibiting the Phosphorylation of JAK2/STAT3 and p38 MAPK in High Glucose-Treated HUVECs.Oxid Med Cell Longev. 2017;2017:9371602. doi: 10.1155/2017/9371602. Epub 2017 Jul 12. Oxid Med Cell Longev. 2017. PMID: 28785380 Free PMC article.
-
Neuroprotective Effects of Ginkgolide B Against Ischemic Stroke: A Review of Current Literature.Curr Top Med Chem. 2015;15(21):2222-32. doi: 10.2174/1568026615666150610142647. Curr Top Med Chem. 2015. PMID: 26059355 Review.
-
Associations between Pregnane X Receptor and Breast Cancer Growth and Progression.Cells. 2020 Oct 15;9(10):2295. doi: 10.3390/cells9102295. Cells. 2020. PMID: 33076284 Free PMC article. Review.
Cited by
-
Role of pregnane X-receptor in regulating bacterial translocation in chronic liver diseases.World J Hepatol. 2017 Nov 18;9(32):1210-1226. doi: 10.4254/wjh.v9.i32.1210. World J Hepatol. 2017. PMID: 29184608 Free PMC article. Review.
-
Comparing the role of Ginkgolide B and Ginkgolide K on cultured astrocytes exposed to oxygen‑glucose deprivation.Mol Med Rep. 2018 Nov;18(5):4417-4427. doi: 10.3892/mmr.2018.9450. Epub 2018 Sep 4. Mol Med Rep. 2018. PMID: 30221704 Free PMC article.
-
Anti-atherosclerotic effects and molecular targets of ginkgolide B from Ginkgo biloba.Acta Pharm Sin B. 2024 Jan;14(1):1-19. doi: 10.1016/j.apsb.2023.09.014. Epub 2023 Sep 23. Acta Pharm Sin B. 2024. PMID: 38239238 Free PMC article. Review.
-
Tanshinone IIA Protects Endothelial Cells from H₂O₂-Induced Injuries via PXR Activation.Biomol Ther (Seoul). 2017 Nov 1;25(6):599-608. doi: 10.4062/biomolther.2016.179. Biomol Ther (Seoul). 2017. PMID: 28173640 Free PMC article.
-
PXR: a center of transcriptional regulation in cancer.Acta Pharm Sin B. 2020 Feb;10(2):197-206. doi: 10.1016/j.apsb.2019.06.012. Epub 2019 Jun 29. Acta Pharm Sin B. 2020. PMID: 32082968 Free PMC article. Review.
References
-
- 3Nishimura M, Naito S, Yokoi T. Tissue-specific mRNA expression profiles of human nuclear receptor subfamilies. Drug Metab Pharmacokin 2004; 19: 135–49. - PubMed
-
- 4Kliewer SA, Goodwin B, Willson TM. The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocrine Rev 2002; 23: 687–702. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

