HSP90 activity is required for MLKL oligomerisation and membrane translocation and the induction of necroptotic cell death
- PMID: 26775703
- PMCID: PMC4816171
- DOI: 10.1038/cddis.2015.386
HSP90 activity is required for MLKL oligomerisation and membrane translocation and the induction of necroptotic cell death
Abstract
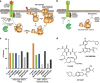
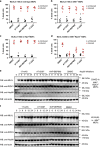
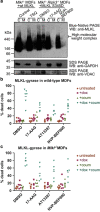
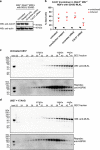
Necroptosis is a caspase-independent form of regulated cell death that has been implicated in the development of a range of inflammatory, autoimmune and neurodegenerative diseases. The pseudokinase, Mixed Lineage Kinase Domain-Like (MLKL), is the most terminal known obligatory effector in the necroptosis pathway, and is activated following phosphorylation by Receptor Interacting Protein Kinase-3 (RIPK3). Activated MLKL translocates to membranes, leading to membrane destabilisation and subsequent cell death. However, the molecular interactions governing the processes downstream of RIPK3 activation remain poorly defined. Using a phenotypic screen, we identified seven heat-shock protein 90 (HSP90) inhibitors that inhibited necroptosis in both wild-type fibroblasts and fibroblasts expressing an activated mutant of MLKL. We observed a modest reduction in MLKL protein levels in human and murine cells following HSP90 inhibition, which was only apparent after 15 h of treatment. The delayed reduction in MLKL protein abundance was unlikely to completely account for defective necroptosis, and, consistent with this, we also found inhibition of HSP90 blocked membrane translocation of activated MLKL. Together, these findings implicate HSP90 as a modulator of necroptosis at the level of MLKL, a function that complements HSP90's previously demonstrated modulation of the upstream necroptosis effector kinases, RIPK1 and RIPK3.
Conflict of interest statement
JS is a member of the Scientific Advisory Board of TetraLogic Pharmaceuticals. GL and JMM lead a programme funded by Catalyst Therapeutics Pty Ltd and the Walter and Eliza Hall Institute to develop necroptosis inhibitors.
Figures

References
-
- Silke J, Rickard JA, Gerlic M. The diverse role of RIP kinases in necroptosis and inflammation. Nat Immunol 2015; 16: 689–697. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

