Intrinsic and chemo-sensitizing activity of SMAC-mimetics on high-risk childhood acute lymphoblastic leukemia
- PMID: 26775704
- PMCID: PMC4816168
- DOI: 10.1038/cddis.2015.382
Intrinsic and chemo-sensitizing activity of SMAC-mimetics on high-risk childhood acute lymphoblastic leukemia
Abstract
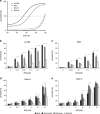
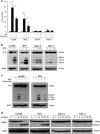
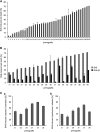
SMAC-mimetics represent a targeted therapy approach to overcome apoptosis resistance in many tumors. Here, we investigated the efficacy of the SMAC-mimetic BV6 in B-cell precursor acute lymphoblastic leukemia (BCP-ALL). In ALL cell lines, intrinsic apoptosis sensitivity was associated with rapid cIAP degradation, NF-κB activation, TNF-α secretion and induction of an autocrine TNF-α-dependent cell death loop. This pattern of responsiveness was also observed upon ex vivo analysis of 40 primograft BCP-ALL samples. Treatment with BV6 induced cell death in the majority of ALL primografts including leukemias with high-risk and poor-prognosis features. Inhibition of cell death by the TNF receptor fusion protein etanercept demonstrated that BV6 activity is dependent on TNF-α. In a preclinical NOD/SCID/huALL model of high-risk ALL, marked anti-leukemia effectivity and significantly prolonged survival were observed upon BV6 treatment. Interestingly, also in vivo, intrinsic SMAC-mimetic activity was mediated by TNF-α. Importantly, BV6 increased the effectivity of conventional induction therapy including vincristine, dexamethasone and asparaginase leading to prolonged remission induction. These data suggest SMAC-mimetics as an important addendum to efficient therapy of pediatric BCP-ALL.
Figures



References
-
- Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med 2006; 354: 166–178. - PubMed
-
- Schrappe M, Reiter A, Ludwig WD, Harbott J, Zimmermann M, Hiddemann W et al. Improved outcome in childhood acute lymphoblastic leukemia despite reduced use of anthracyclines and cranial radiotherapy: results of trial ALL-BFM 90. German-Austrian-Swiss ALL-BFM Study Group. Blood 2000; 95: 3310–3322. - PubMed
-
- Stanulla M, Cario G, Meissner B, Schrauder A, Moricke A, Riehm H et al. Integrating molecular information into treatment of childhood acute lymphoblastic leukemia—a perspective from the BFM Study Group. Blood Cells Mol Dis 2007; 39: 160–163. - PubMed
-
- Fulda S. Tumor resistance to apoptosis. Int J Cancer 2009; 124: 511–515. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

