Effect of hydroxychloroquine and characterization of autophagy in a mouse model of endometriosis
- PMID: 26775710
- PMCID: PMC4816166
- DOI: 10.1038/cddis.2015.361
Effect of hydroxychloroquine and characterization of autophagy in a mouse model of endometriosis
Abstract
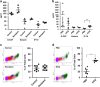
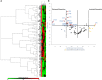
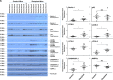
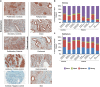
In endometriosis, the increased survival potential of shed endometrial cells (which normally undergo anoikis) is suggested to promote lesion development. One mechanism that may alter anoikis is autophagy. Using an autophagic flux inhibitor hydroxychloroquine (HCQ), we identified that it reduces the in vitro survival capacity of human endometriotic and endometrial T-HESC cells. We also identified that HCQ could decrease lesion numbers and disrupt lesion histopathology, as well as increase the levels of peritoneal macrophages and the IP-10 (10 kDa interferon-γ-induced protein) chemokine in a mouse model of endometriosis. We noted that RNA levels of a subset of autophagic markers were reduced in lesions relative to uterine horns from endometriosis-induced (untreated) mice. In addition, the RNA levels of autophagic markers were decreased in uterine horns of endometriosis-induced mice compared with those from controls. However, we noted that protein expression of LC3B (microtubule-associated protein 1 light-chain 3β; an autophagic marker) was increased in uterine horns of endometriosis-induced mice compared with uterine horns of controls. By immunohistochemical staining of a human endometriosis-focused tissue microarray, we observed LC3B expression predominantly in epithelial relative to stromal cells in both eutopic and ectopic endometria. Via transmission electron microscopy, cells from eutopic endometria of endometriosis-induced mice contained more lipid droplets (rather than autophagosomes) compared with uterine horns from controls. Collectively, our findings indicate that the autophagic pathway is dysregulated in both ectopic and eutopic endometrium in a murine model of endometriosis and that HCQ has potential as a therapeutic agent for women afflicted with endometriosis.
Conflict of interest statement
A provisional patent application on autophagy and endometriosis has been submitted” (MN, IF). All other co-authors declare no conflict of interest.
Figures








Similar articles
-
An Observation of the Role of Autophagy in Patients with Endometriosis of Different Stages during Secretory Phase and Proliferative Phase.Curr Gene Ther. 2018;18(5):286-295. doi: 10.2174/1566523218666181008155039. Curr Gene Ther. 2018. PMID: 30306868
-
Alteration of Nrf2 and Glutamate Cysteine Ligase expression contribute to lesions growth and fibrogenesis in ectopic endometriosis.Free Radic Biol Med. 2017 Sep;110:1-10. doi: 10.1016/j.freeradbiomed.2017.04.362. Epub 2017 Apr 28. Free Radic Biol Med. 2017. PMID: 28457937
-
[Lipoxin A4 inhibits the invasion and migration of endometrial stromal cells by down-regulating NF-κB signaling-mediated autophagy].Zhonghua Fu Chan Ke Za Zhi. 2018 Aug 25;53(8):547-553. doi: 10.3760/cma.j.issn.0529-567x.2018.08.007. Zhonghua Fu Chan Ke Za Zhi. 2018. PMID: 30138965 Chinese.
-
Formononetin Inhibits Progression of Endometriosis via Regulation of p27, pSTAT3, and Progesterone Receptor: In Vitro and In Vivo Studies.Nutrients. 2023 Jun 30;15(13):3001. doi: 10.3390/nu15133001. Nutrients. 2023. PMID: 37447325 Free PMC article.
-
Cancer-associated mutations in endometriosis: shedding light on the pathogenesis and pathophysiology.Hum Reprod Update. 2020 Apr 15;26(3):423-449. doi: 10.1093/humupd/dmz047. Hum Reprod Update. 2020. PMID: 32154564 Review.
Cited by
-
Increased Expression of YAP Inhibited the Autophagy Level by Upregulating mTOR Signal in the Eutopic ESCs of Endometriosis.Front Endocrinol (Lausanne). 2022 Jan 31;13:813165. doi: 10.3389/fendo.2022.813165. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35173685 Free PMC article.
-
Hyaluronic Acid-Modified Nanoplatforms as a Vector for Targeted Delivery of Autophagy-Related Gene to the Endometriotic Lesions in Mice.Front Bioeng Biotechnol. 2022 Jul 1;10:918368. doi: 10.3389/fbioe.2022.918368. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35845410 Free PMC article.
-
Hypo-Expression of Tuberin Promotes Adenomyosis via the mTOR1-Autophagy Axis.Front Cell Dev Biol. 2021 Jul 29;9:710407. doi: 10.3389/fcell.2021.710407. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34395438 Free PMC article.
-
Recent Clinical and Preclinical Studies of Hydroxychloroquine on RNA Viruses and Chronic Diseases: A Systematic Review.Molecules. 2020 Nov 14;25(22):5318. doi: 10.3390/molecules25225318. Molecules. 2020. PMID: 33202656 Free PMC article.
-
Preclinical models of endometriosis and interstitial cystitis/bladder pain syndrome: an Innovative Medicines Initiative-PainCare initiative to improve their value for translational research in pelvic pain.Pain. 2021 Sep 1;162(9):2349-2365. doi: 10.1097/j.pain.0000000000002248. Pain. 2021. PMID: 34448751 Free PMC article. Review.
References
-
- Giudice LC, Kao LC. Endometriosis. Lancet 2004; 364: 1789–1799. - PubMed
-
- Bulun SE. Endometriosis. N Engl J Med 2009; 360: 268–279. - PubMed
-
- Sampson JA. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol 1927; 14: 422–469. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

