Common mutations in ALK2/ACVR1, a multi-faceted receptor, have roles in distinct pediatric musculoskeletal and neural orphan disorders
- PMID: 26776312
- PMCID: PMC4753137
- DOI: 10.1016/j.cytogfr.2015.12.007
Common mutations in ALK2/ACVR1, a multi-faceted receptor, have roles in distinct pediatric musculoskeletal and neural orphan disorders
Abstract
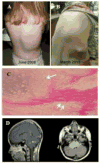
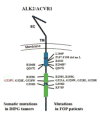
Activin receptor-like kinase-2 (ALK2), the product of ACVR1, is a member of the type I bone morphogenetic protein (BMP) receptor family. ALK2 exerts key and non-redundant roles in numerous developmental processes, including the specification, growth and morphogenesis of endochondral skeletal elements. There is also strong evidence that BMP signaling plays important roles in determination, differentiation and function of neural cells and tissues. Here we focus on the intriguing discovery that common activating mutations in ALK2 occur in Fibrodysplasia Ossificans Progressiva (FOP) and Diffuse Intrinsic Pontine Gliomas (DIPGs), distinct pediatric disorders of significant severity that are associated with premature death. Pathogenesis and treatment remain elusive for both. We consider recent studies on the nature of the ACVR1 mutations, possible modes of action and targets, and plausible therapeutic measures. Comparisons of the diverse - but genetically interrelated - pathologies of FOP and DIPG will continue to be of major mutual benefit with broad biomedical and clinical relevance.
Keywords: ACVR1; ALK2; BMP signaling; Diffuse Intrinsic Pontine Gliomas; Fibrodysplasia Ossificans Progressiva; Orphan diseases.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Conflict of interest statement
One of the authors (MP) is a consultant for Clementia Pharmaceuticals.
Figures

Similar articles
-
Effects of FKBP12 and type II BMP receptors on signal transduction by ALK2 activating mutations associated with genetic disorders.Bone. 2018 Jun;111:101-108. doi: 10.1016/j.bone.2018.03.015. Epub 2018 Mar 15. Bone. 2018. PMID: 29551750
-
Shared ACVR1 mutations in FOP and DIPG: Opportunities and challenges in extending biological and clinical implications across rare diseases.Bone. 2018 Apr;109:91-100. doi: 10.1016/j.bone.2017.08.001. Epub 2017 Aug 2. Bone. 2018. PMID: 28780023 Free PMC article.
-
ALK2: A Therapeutic Target for Fibrodysplasia Ossificans Progressiva and Diffuse Intrinsic Pontine Glioma.Chem Pharm Bull (Tokyo). 2020;68(3):194-200. doi: 10.1248/cpb.c19-00882. Chem Pharm Bull (Tokyo). 2020. PMID: 32115526 Review.
-
ACVR1 Function in Health and Disease.Cells. 2019 Oct 31;8(11):1366. doi: 10.3390/cells8111366. Cells. 2019. PMID: 31683698 Free PMC article. Review.
-
ACVR1 mutations in DIPG: lessons learned from FOP.Cancer Res. 2014 Sep 1;74(17):4565-70. doi: 10.1158/0008-5472.CAN-14-1298. Epub 2014 Aug 18. Cancer Res. 2014. PMID: 25136070 Free PMC article. Review.
Cited by
-
Drosophila models of FOP provide mechanistic insight.Bone. 2018 Apr;109:192-200. doi: 10.1016/j.bone.2017.11.001. Epub 2017 Nov 8. Bone. 2018. PMID: 29128351 Free PMC article.
-
iMSC-mediated delivery of ACVR2B-Fc fusion protein reduces heterotopic ossification in a mouse model of fibrodysplasia ossificans progressiva.Stem Cell Res Ther. 2024 Mar 18;15(1):83. doi: 10.1186/s13287-024-03691-7. Stem Cell Res Ther. 2024. PMID: 38500216 Free PMC article.
-
Cutting Edge Therapeutic Insights Derived from Molecular Biology of Pediatric High-Grade Glioma and Diffuse Intrinsic Pontine Glioma (DIPG).Bioengineering (Basel). 2018 Oct 18;5(4):88. doi: 10.3390/bioengineering5040088. Bioengineering (Basel). 2018. PMID: 30340362 Free PMC article. Review.
-
Intersections of Fibrodysplasia Ossificans Progressiva and Traumatic Heterotopic Ossification.Biomolecules. 2024 Mar 14;14(3):349. doi: 10.3390/biom14030349. Biomolecules. 2024. PMID: 38540768 Free PMC article. Review.
-
Targeting ALK2: An Open Science Approach to Developing Therapeutics for the Treatment of Diffuse Intrinsic Pontine Glioma.J Med Chem. 2020 May 14;63(9):4978-4996. doi: 10.1021/acs.jmedchem.0c00395. Epub 2020 May 5. J Med Chem. 2020. PMID: 32369358 Free PMC article.
References
-
- Schmierer B, Hill CS. TGFβ-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol. 2007;8:970–82. - PubMed
-
- Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233–41. - PubMed
-
- Clarke TR, Hoshiya Y, Yi SE, Liu X, Lyons KM, Donahoe PK. Mullerian inhibiting substance signaling uses a bone morphogenetic protein (BMP)-like pathway mediated by ALK2 and induces SMAD6 expression. Mol Endocrinology. 2001;15:946–59. - PubMed
-
- Zhang D, Schwarz EM, Rosier RN, Zuscik MJ, Puzas JE, O’Keefe RJ. ALK2 functions as a BMP type I receptor and induces Indian hedgehog in chondrocytes during skeletal development. J Bone Min Res. 2003;18:1593–604. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

