Comparisons of dysphagia and quality of life (QOL) in comparable patients with HPV-positive oropharyngeal cancer receiving chemo-irradiation or cetuximab-irradiation
- PMID: 26776757
- PMCID: PMC4754145
- DOI: 10.1016/j.oraloncology.2015.12.001
Comparisons of dysphagia and quality of life (QOL) in comparable patients with HPV-positive oropharyngeal cancer receiving chemo-irradiation or cetuximab-irradiation
Abstract
Purpose: Compare functional outcomes of radiotherapy (RT) concurrent with cetuximab (cet-RT) or with chemotherapy (chemo-RT) for comparable, good prognosis patients with human papillomavirus related (HPV+) oropharyngeal cancer (OPC).
Methods: Outcomes of patients with stage III/IV HPV+ OPC patients with minimal smoking history and non-T4/N3/N2C, treated on prospective protocol of RT concurrent with cetuximab (cet-RT), were compared to similar patients on prospective chemo-RT protocols. In both groups, videofluoroscopy (VF), observer rated dysphagia (ORD), and validated QOL questionnaires: xerostomia questionnaire (XQ), head and neck QOL, and University of Washington QOL, were performed periodically and compared to pretreatment. Mixed effects models with adjustment for baseline assessed differences between groups.
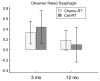
Results: 26 cet-RT patients were compared to 27 chemo-RT patients with similar baseline characteristics. In the chemo-RT group, no recurrences occurred. In the cet-RT group, 1 patient had persistent microscopic disease on salvage neck dissection and 1 distant failure. Both groups had mild VF-based swallowing dysfunction pre-treatment, worsened at 3 months (P<0.02) and persisted at 12 months, not differing between groups (P>0.11). For both groups ORD was very low pretreatment, worsened at 3 months and improved at 12 months, without differences between treatment groups (P=0.26). QOL Summary and domain scores for eating were good pretreatment, worse at 3 mo, and then improved to near baseline at 12 months, without differences between the groups in any QOL domains (P>0.10).
Conclusion: Both groups had excellent clinical outcomes without significant differences in objective or subjective functions. These data question using cetuximab instead of chemotherapy for treatment de-intensification for HPV+ OPC.
Keywords: Cetuximab; Chemo-RT; Oropharyngeal cancer; Quality of life; Swallowing.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest statement: None of the authors has conflict of interest.
Figures


References
-
- Ang KK, Sturgis EM. Semin radiat oncol. United States: A 2012 Elsevier Inc; 2012. Human papillomavirus as a marker of the natural history and response to therapy of head and neck squamous cell carcinoma; pp. 128–42. - PubMed
-
- O’Sullivan B, Huang SH, Siu LL, et al. Deintensification candidate subgroups in human papillomavirus-related oropharyngeal cancer according to minimal risk of distant metastasis. J Clin Oncol. 2013;31:543–50. - PubMed
-
- Masterson L, Moualed D, Liu ZW, et al. De-escalation treatment protocols for human papillomavirus-associated oropharyngeal squamous cell carcinoma: A systematic review and meta-analysis of current clinical trials. Eur J Cancer. 2014;50:2636–2648. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

