E-cigarettes and smoking cessation in real-world and clinical settings: a systematic review and meta-analysis
- PMID: 26776875
- PMCID: PMC4752870
- DOI: 10.1016/S2213-2600(15)00521-4
E-cigarettes and smoking cessation in real-world and clinical settings: a systematic review and meta-analysis
Abstract
Background: Smokers increasingly use e-cigarettes for many reasons, including attempts to quit combustible cigarettes and to use nicotine where smoking is prohibited. We aimed to assess the association between e-cigarette use and cigarette smoking cessation among adult cigarette smokers, irrespective of their motivation for using e-cigarettes.
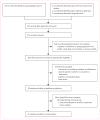
Methods: PubMed and Web of Science were searched between April 27, 2015, and June 17, 2015. Data extracted included study location, design, population, definition and prevalence of e-cigarette use, comparison group (if applicable), cigarette consumption, level of nicotine dependence, other confounders, definition of quitting smoking, and odds of quitting smoking. The primary endpoint was cigarette smoking cessation. Odds of smoking cessation among smokers using e-cigarettes compared with smokers not using e-cigarettes were assessed using a random effects meta-analysis. A modification of the ACROBAT-NRSI tool and the Cochrane Risk of Bias Tool were used to assess bias. This meta-analysis is registered with PROSPERO (number CRD42015020382).
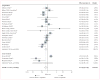
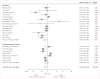
Findings: 38 studies (of 577 studies identified) were included in the systematic review; all 20 studies with control groups (15 cohort studies, three cross-sectional studies, and two clinical trials) were included in random effects meta-analysis and sensitivity analyses. Odds of quitting cigarettes were 28% lower in those who used e-cigarettes compared with those who did not use e-cigarettes (odds ratio [OR] 0·72, 95% CI 0·57-0·91). Association of e-cigarette use with quitting did not significantly differ among studies of all smokers using e-cigarettes (irrespective of interest in quitting cigarettes) compared with studies of only smokers interested in cigarette cessation (OR 0·63, 95% CI 0·45-0·86 vs 0·86, 0·60-1·23; p=0·94). Other study characteristics (design, population, comparison group, control variables, time of exposure assessment, biochemical verification of abstinence, and definition of e-cigarette use) were also not associated with the overall effect size (p≥0·77 in all cases).
Interpretation: As currently being used, e-cigarettes are associated with significantly less quitting among smokers.
Funding: National Institutes of Health, National Cancer Institute, FDA Center for Tobacco Products.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Conflict of interest statement
SAG is Truth Initiative Foundation Distinguished Professor of Tobacco Control. We declare no competing interests.
Figures
Comment in
-
Electronic cigarettes: more light, less heat needed.Lancet Respir Med. 2016 Feb;4(2):85-7. doi: 10.1016/S2213-2600(16)00010-2. Epub 2016 Jan 14. Lancet Respir Med. 2016. PMID: 26776876 No abstract available.
-
E-cigarettes and smoking cessation - Authors' reply.Lancet Respir Med. 2016 Jun;4(6):e26-7. doi: 10.1016/S2213-2600(16)30025-X. Epub 2016 Apr 25. Lancet Respir Med. 2016. PMID: 27133215 No abstract available.
-
E-cigarettes and smoking cessation.Lancet Respir Med. 2016 Jun;4(6):e23. doi: 10.1016/S2213-2600(16)30024-8. Epub 2016 Apr 25. Lancet Respir Med. 2016. PMID: 27133216 No abstract available.
-
E-cigarettes and smoking cessation.Lancet Respir Med. 2016 Jun;4(6):e25. doi: 10.1016/S2213-2600(16)30021-2. Epub 2016 Apr 25. Lancet Respir Med. 2016. PMID: 27133217 No abstract available.
-
E-cigarettes and smoking cessation.Lancet Respir Med. 2016 Jun;4(6):e24. doi: 10.1016/S2213-2600(16)30023-6. Epub 2016 Apr 25. Lancet Respir Med. 2016. PMID: 27133218 No abstract available.
References
-
- Action on Smoking and Health. Use of electronic cigarettes (vapourisers) among adults in Great Britain. [accessed June 5, 2015];ASH fact sheet. http://www.ash.org.uk/files/documents/ASH_891.pdf.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

