Aqueous Hydricity of Late Metal Catalysts as a Continuum Tuned by Ligands and the Medium
- PMID: 26777267
- PMCID: PMC4768292
- DOI: 10.1021/jacs.5b12363
Aqueous Hydricity of Late Metal Catalysts as a Continuum Tuned by Ligands and the Medium
Abstract
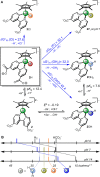
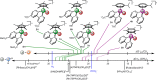
Aqueous hydride transfer is a fundamental step in emerging alternative energy transformations such as H2 evolution and CO2 reduction. "Hydricity," the hydride donor ability of a species, is a key metric for understanding transition metal hydride reactivity, but comprehensive studies of aqueous hydricity are scarce. An extensive and self-consistent aqueous hydricity scale is constructed for a family of Ru and Ir hydrides that are key intermediates in aqueous catalysis. A reference hydricity is determined using redox potentiometry and spectrophotometric titration for a particularly water-soluble species. Then, relative hydricity values for a range of species are measured using hydride transfer equilibria, taking advantage of expedient new synthetic procedures for Ru and Ir hydrides. This large collection of hydricity values provides the most comprehensive picture so far of how ligands impact hydricity in water. Strikingly, we also find that hydricity can be viewed as a continuum in water: the free energy of hydride transfer changes with pH, buffer composition, and salts present in solution.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

