Melanoma Immunotherapy in Mice Using Genetically Engineered Pluripotent Stem Cells
- PMID: 26777320
- PMCID: PMC5538580
- DOI: 10.3727/096368916X690467
Melanoma Immunotherapy in Mice Using Genetically Engineered Pluripotent Stem Cells
Abstract
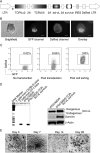
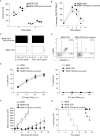
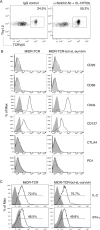
Adoptive cell transfer (ACT) of antigen (Ag)-specific CD8(+) cytotoxic T lymphocytes (CTLs) is a highly promising treatment for a variety of diseases. Naive or central memory T-cell-derived effector CTLs are optimal populations for ACT-based immunotherapy because these cells have a high proliferative potential, are less prone to apoptosis than terminally differentiated cells, and have the higher ability to respond to homeostatic cytokines. However, such ACT with T-cell persistence is often not feasible due to difficulties in obtaining sufficient cells from patients. Here we present that in vitro differentiated HSCs of engineered PSCs can develop in vivo into tumor Ag-specific naive CTLs, which efficiently suppress melanoma growth. Mouse-induced PSCs (iPSCs) were retrovirally transduced with a construct encoding chicken ovalbumin (OVA)-specific T-cell receptors (TCRs) and survival-related proteins (i.e., BCL-xL and survivin). The gene-transduced iPSCs were cultured on the delta-like ligand 1-expressing OP9 (OP9-DL1) murine stromal cells in the presence of murine recombinant cytokines (rFlt3L and rIL-7) for a week. These iPSC-derived cells were then intravenously adoptively transferred into recipient mice, followed by intraperitoneal injection with an agonist α-Notch 2 antibody and cytokines (rFlt3L and rIL-7). Two weeks later, naive OVA-specific CD8(+) T cells were observed in the mouse peripheral lymphatic system, which were responsive to OVA-specific stimulation. Moreover, the mice were resistant to the challenge of B16-OVA melanoma induction. These results indicate that genetically modified stem cells may be used for ACT-based immunotherapy or serve as potential vaccines.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
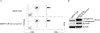
Similar articles
-
In vivo programming of tumor antigen-specific T lymphocytes from pluripotent stem cells to promote cancer immunosurveillance.Cancer Res. 2011 Jul 15;71(14):4742-7. doi: 10.1158/0008-5472.CAN-11-0359. Epub 2011 May 31. Cancer Res. 2011. PMID: 21628492
-
Directed differentiation of induced pluripotent stem cells towards T lymphocytes.J Vis Exp. 2012 May 14;(63):e3986. doi: 10.3791/3986. J Vis Exp. 2012. PMID: 22617911 Free PMC article.
-
Generation of Tumor Antigen-Specific Cytotoxic T Lymphocytes from Pluripotent Stem Cells.Methods Mol Biol. 2019;1884:43-55. doi: 10.1007/978-1-4939-8885-3_3. Methods Mol Biol. 2019. PMID: 30465194 Free PMC article.
-
The Anticancer Potential of T Cell Receptor-Engineered T Cells.Trends Cancer. 2021 Jan;7(1):48-56. doi: 10.1016/j.trecan.2020.09.002. Epub 2020 Sep 26. Trends Cancer. 2021. PMID: 32988787 Free PMC article. Review.
-
Pluripotent stem cell-derived natural killer cells for cancer therapy.Transl Res. 2010 Sep;156(3):147-54. doi: 10.1016/j.trsl.2010.07.008. Epub 2010 Aug 1. Transl Res. 2010. PMID: 20801411 Free PMC article. Review.
Cited by
-
Adoptive Cell Transfer: Is it a Promising Immunotherapy for Colorectal Cancer?Theranostics. 2018 Nov 10;8(20):5784-5800. doi: 10.7150/thno.29035. eCollection 2018. Theranostics. 2018. PMID: 30555581 Free PMC article. Review.
-
Predictive value of TCR Vβ-Jβ profile for adjuvant gefitinib in EGFR mutant NSCLC from ADJUVANT-CTONG 1104 trial.JCI Insight. 2022 Jan 11;7(1):e152631. doi: 10.1172/jci.insight.152631. JCI Insight. 2022. PMID: 35014626 Free PMC article.
-
Development of Auto Antigen-specific Regulatory T Cells for Diabetes Immunotherapy.Immune Netw. 2016 Oct;16(5):281-285. doi: 10.4110/in.2016.16.5.281. Epub 2016 Oct 25. Immune Netw. 2016. PMID: 27799873 Free PMC article. Review.
-
Genomic and T cell repertoire biomarkers associated with malignant mesothelioma survival.Thorac Cancer. 2024 Jul;15(19):1502-1512. doi: 10.1111/1759-7714.15326. Epub 2024 May 27. Thorac Cancer. 2024. PMID: 38798202 Free PMC article.
-
Protective Cancer Vaccine Using Genetically Modified Hematopoietic Stem Cells.Vaccines (Basel). 2018 Jul 6;6(3):40. doi: 10.3390/vaccines6030040. Vaccines (Basel). 2018. PMID: 29986440 Free PMC article.
References
-
- Bargou R, Leo E, Zugmaier G, Klinger M, Goebeler M, Knop S, Noppeney R, Viardot A, Hess G, Schuler M, Einsele H, Brandl C, Wolf A, Kirchinger P, Klappers P, Schmidt M, Riethmuller G, Reinhardt C, Baeuerle PA, Kufer P. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321(5891):974–977. - PubMed
-
- Bartlett EK, Fetsch PA, Filie AC, Abati A, Steinberg SM, Wunderlich JR, White DE, Stephens DJ, Marincola FM, Rosenberg SA, Kammula US. Human melanoma metastases demonstrate nonstochastic site-specific antigen heterogeneity that correlates with T-cell infiltration. Clin. Cancer Res. 2014;20(10):2607–2616. - PMC - PubMed
-
- Bendle GM, Linnemann C, Hooijkaas AI, Bies L, de Witte MA, Jorritsma A, Kaiser AD, Pouw N, Debets R, Kieback E, Uckert W, Song JY, Haanen JB, Schumacher TN. Lethal graft-versus-host disease in mouse models of T cell receptor gene therapy. Nat. Med. 2010;16(5):565–570. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

